Copper homeostasis and cuproptosis in Alzheimer's disease (Review)
- PMID: 40849807
- PMCID: PMC12404895
- DOI: 10.3892/ijmm.2025.5613
Copper homeostasis and cuproptosis in Alzheimer's disease (Review)
Abstract
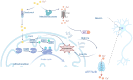
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by neuroinflammation, synaptic dysfunction and neuronal loss. Research has revealed a connection between copper metabolism and the pathophysiology of AD, particularly through a newly identified form of copper‑dependent cell death referred to as cuproptosis. Cuproptosis is driven by the aggregation of lipoylated proteins and proteotoxic stress caused by excessive copper accumulation, leading to cellular demise, which is a key event in AD. While studies on copper levels in the brain in AD remain inconclusive, there is mounting evidence suggesting that an imbalance in copper homeostasis, particularly elevated labile copper levels, contributes to oxidative damage and neurodegeneration in patients with AD. The present review examines the role of cuproptosis in AD and discusses how targeting this pathway may provide novel therapeutic opportunities. By investigating the underlying mechanisms and potential clinical implications, the present review highlights that regulation of cuproptosis provides a promising approach for modulating disease progression and developing personalized treatment strategies for AD.
Keywords: Alzheimer's disease; cuproptosis; neuroinflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Copper metabolism and cuproptosis in Alzheimer's disease: mechanisms and therapeutic potential.Biomed Pharmacother. 2025 Sep;190:118354. doi: 10.1016/j.biopha.2025.118354. Epub 2025 Jul 17. Biomed Pharmacother. 2025. PMID: 40680673 Review.
-
Cuproptosis: a novel therapeutic mechanism in lung cancer.Cancer Cell Int. 2025 Jun 24;25(1):231. doi: 10.1186/s12935-025-03864-1. Cancer Cell Int. 2025. PMID: 40555995 Free PMC article. Review.
-
Cuproptosis: Mechanisms, biological significance, and advances in disease treatment-A systematic review.CNS Neurosci Ther. 2024 Sep;30(9):e70039. doi: 10.1111/cns.70039. CNS Neurosci Ther. 2024. PMID: 39267265 Free PMC article.
-
Cell-specific copper dyshomeostasis mechanism in Alzheimer's disease.Transl Neurodegener. 2025 Aug 22;14(1):42. doi: 10.1186/s40035-025-00504-6. Transl Neurodegener. 2025. PMID: 40847318 Free PMC article. Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
References
-
- Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc Natl Acad Sci USA. 2011;108:5980–5985. doi: 10.1073/pnas.1009932108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
