Current status of severe fever with thrombocytopenia syndrome in China (Review)
- PMID: 40849814
- PMCID: PMC12373438
- DOI: 10.3892/ijmm.2025.5610
Current status of severe fever with thrombocytopenia syndrome in China (Review)
Abstract
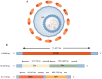
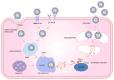
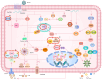
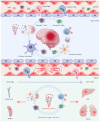
Severe fever with thrombocytopenia syndrome (SFTS) is a newly emerging tick‑borne infectious disease caused by the novel Bunyavirus/SFTS virus (SFTSV). The clinical manifestations mainly include fever, thrombocytopenia and multi‑organ dysfunction, with a fatality rate as high as 30%. Since its first report in China in 2009, cases have subsequently emerged in multiple countries across East and Southeast Asia. SFTS demonstrates clear seasonal trends from May to November and tends to cluster geographically, mainly in hilly and mountainous areas. The virus is transmitted through tick bites, animal contact and human‑to‑human transmission. Its genetic diversity and frequent genetic recombination exacerbate public health threats. Pathogenic mechanism studies have shown that SFTSV uses glycoproteins Gn/Gc to mediate host cell invasion. In the early stage, the virus uses its non‑structural protein NSs to inhibit innate immune signal transduction. Massive replication of the virus leads to excessive immune activation, triggering cytokine storms and abnormal platelet activation, and eventually resulting in bleeding and multiple organ failure. The clinical management primarily relies on supportive care, while broad‑spectrum antiviral drugs and neutralizing antibodies remain investigational. Although numerous vaccine candidates have been designed and developed, none have progressed to clinical trials. This review systematically integrates current knowledge spanning virology, epidemiology, pathogenic mechanisms, therapeutic interventions and vaccine development, offering actionable insights for public health strategies and clinical practice.
Keywords: SFTS; SFTSV; epidemiological characteristics; etiological characteristics; pathogenic mechanism; treatment; vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
