Ocular changes as potential biomarkers for early diagnosis of Alzheimer's disease
- PMID: 40851106
- PMCID: PMC12375435
- DOI: 10.1002/alz.70476
Ocular changes as potential biomarkers for early diagnosis of Alzheimer's disease
Abstract
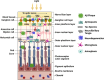
Alzheimer's disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia. AD diagnosis often involves a thorough assessment, including clinical evaluation, cognitive testing, medical history examination, genetic testing, and biomarker analysis. Currently, the invasive nature and high costs of biomarker testing, such as cerebrospinal fluid analysis and neuroimaging, have limited the early detection and intervention of the disease. Therefore, researchers have explored cheaper and less invasive options, such as ocular imaging. This review discusses the close relationship between the eye and the central nervous system and ocular changes as a potential non-invasive AD biomarker. Ocular changes in AD have frequently been reported in the literature, particularly in the later stages of the disease. However, identifying biomarkers specifically attributable to AD remains a challenge. Additionally, this review provides a comprehensive overview of existing studies and highlights potential pathways for enhancing AD detection through ocular structural and functional evaluation. HIGHLIGHTS: Widespread screening for early detection of Alzheimer's disease (AD) is limited by cost and time constraints with current methods. Non-invasive and inexpensive methods are needed to overcome these difficulties. The eye is readily accessible and has shared developmental origins, neurobiology, and neurochemistry with the brain. The expanding field of ocular biomarker studies needs larger, well-characterized cohorts and longitudinal studies to understand the ocular changes for preclinical AD screening. Standards should be established for more methodical investigative approaches to identify ocular biomarkers that are specifically attributable to AD.
Keywords: Alzheimer's disease; amyloid beta; biomarkers; brain; early detection; ocular; retina.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures


References
-
- WHO . Dementia. World Health Organization. 31 March 2025. Accessed July 15, 2025. https://www.who.int/news‐room/fact‐sheets/detail/dementia
-
- Alzheimer's Association . 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. - PubMed
-
- A Armstrong R. Risk factors for Alzheimer's disease. Folia Neuropathol. 2019;57(2):87‐105. - PubMed
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939‐939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

