Expression Diversity in Endocrine Cells and Between Species Revealed by Novel Synthetic Peptide Antibodies Recognizing the Neuroendocrine Protein 7B2
- PMID: 40851497
- PMCID: PMC12378109
- DOI: 10.1369/00221554251365996
Expression Diversity in Endocrine Cells and Between Species Revealed by Novel Synthetic Peptide Antibodies Recognizing the Neuroendocrine Protein 7B2
Abstract
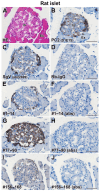
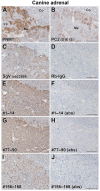
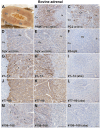
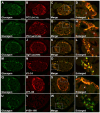
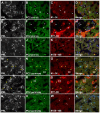
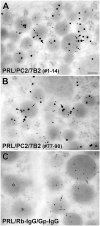
The neuroendocrine protein 7B2 plays a crucial role in the maturation and activity regulation of prohormone convertase 2 (PC2). To elucidate the relationship between 7B2 and PC2 expression in endocrine tissues, we generated synthetic peptide antibodies in guinea pigs. The antigenic peptide sequences were selected to correspond to three different positions in the rat amino acid (aa) sequence: The N-terminal aa 1-14 is situated immediately following the signal sequence, the middle aa 77-90 contains a part of the proPC2 activation domain, and the C-terminal aa 156-168 functions to suppress PC2 activity. These antibodies demonstrated specific reactivity across a diverse array of animal species. The reactivity of these antibodies differed, suggesting that the molecular form of 7B2 differs depending on the endocrine cell, and a different expression pattern was demonstrated in rat and dog pituitary intermediate cells. The colocalization of 7B2 and PC2 in prolactin (PRL) granules in rat pituitary mammotrophs supports the interaction between these proteins. However, the expression intensities of these proteins did not correspond, and epitope-related disparities were detected. These results may be indicative of alterations in the molecular state associated with the dynamics of the interaction between 7B2 and PC2.
Keywords: adrenal medulla; gastrointestinal endocrine cell; pancreatic islets; peptide hormone; pituitary intermediate lobe; processing enzyme; secretogranin V; thyroid gland.
Conflict of interest statement
Competing InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







References
-
- Hsi KL, Seidah NG, De Serres G, Chrétien M. Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett. 1982;147(2):261–6. - PubMed
-
- Seidah NG, Hsi KL, De Serres G, Rochemont J, Hamelin J, Antakly T, Cantin M, Chrétien M. Isolation and NH2-terminal sequence of a highly conserved human and porcine pituitary protein belonging to a new superfamily. Immunocytochemical localization in pars distalis and pars nervosa of the pituitary and in the supraoptic nucleus of the hypothalamus. Arch Biochem Biophys. 1983;225(2):525–34. - PubMed
-
- Iguchi H, Chan JS, Seidah NG, Chrétien M. Tissue distribution and molecular forms of a novel pituitary protein in the rat. Neuroendocrinology. 1984;39(5):453–8. - PubMed
-
- Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348(12):1134–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

