Potential Process Control Issues With Abatacept
- PMID: 40851918
- PMCID: PMC12370286
Potential Process Control Issues With Abatacept
Abstract
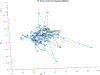
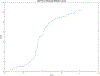
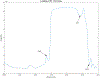
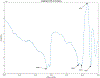
Abatacept is a medication administered through intravenous infusion. It is supplied as a sterile, white, preservative-free, freeze-dried powder. Each vial of drug contains 250 mg abatacept, maltose, monobasic sodium phosphate, and sodium chloride for administration. Abatacept is a fusion protein consisting of the extracellular domain of CTLA-4 linked to the modified Fc portion of human immunoglobulin G1. It is produced using recombinant DNA technology. Abatacept is indicated for moderately to severely active rheumatoid arthritis in adults and polyarticular juvenile idiopathic arthritis in pediatric patients 6 years of age and older. It can be used as monotherapy or in combination with other disease-modifying antirheumatic drugs or methotrexate. Inter-lot variability was detected in a library of 132 vials spread across 34 lots of abatacept-maltose for injection by the University of Kentucky Drug Quality Task Force. A subcluster detection test was run on 13 vials that were shown to be an outlier group (rtn=0.9940, rtest=0.9551, rlim=0.9865, p=0.02). Five of these vials individually appeared 4 or more standard deviations from the library cluster.
Figures

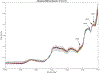

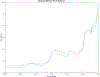

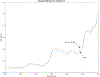
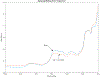

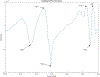
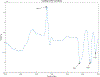
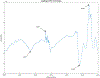
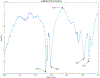
References
-
- BMS, 2021. Orencia “Highlights Of Prescribing Information,” https://packageinserts.bms.com/pi/pi_orencia.pdf, retrieved Aug. 28, 2023.
-
- Dempsey RJ, Davis DG, Buice RG Jr, & Lodder RA (1996). Biological and medical applications of near-infrared spectrometry. Applied Spectroscopy, 50(2), 18A–34A.
-
- Drug Shortages Canada (2021). https://www.drugshortagescanada.ca/shortage/146256. Retrieved Aug. 20, 2023
-
- FDA, 2013. Orencia “Highlights Of Prescribing Information” https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125118s171lbl.pdf, Retrieved Aug. 20, 2023
Grants and funding
LinkOut - more resources
Full Text Sources
