The pump, the exchanger, and the Holy Spirit: tracing the 40-year evolution of the Ouabain-Na+ pump endocrine system concept
- PMID: 40851957
- PMCID: PMC12369745
- DOI: 10.1097/MS9.0000000000003438
The pump, the exchanger, and the Holy Spirit: tracing the 40-year evolution of the Ouabain-Na+ pump endocrine system concept
Abstract
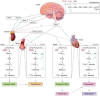
The discovery and subsequent evolution of the Ouabain-Na+/K+ pump endocrine system have profoundly impacted our understanding of cellular physiology and disease mechanisms. Initially identified as a cardiotonic steroid with potent effects on the Na+/K+ ATPase, Ouabain has been implicated in various physiological and pathological processes. The Na+/K+ pump, a crucial component of cellular physiology, maintains electrochemical gradients essential for nerve impulse transmission, muscle contraction, and cellular volume regulation. Since Jens Christian Skou's Nobel Prize-winning discovery in 1957, research has unveiled its broader role in cellular homeostasis and disease. A significant breakthrough was the identification of Ouabain as an endogenous ligand of the Na+/K+ pump, transforming the pump's role from a mere ion transporter to a receptor within a hormonal signaling pathway. This discovery has linked the Na+/K+ pump to complex intracellular signaling pathways, with implications for hypertension, heart failure, and cancer. However, emerging evidence suggests that its role extends beyond cardiovascular dysfunction to neurological disorders such as epilepsy, Alzheimer's disease, and Parkinson's disease. In epilepsy, dysregulation of the Na+/K+ pump contributes to altered ion homeostasis and hyperexcitability. At the same time, in Alzheimer's disease, its dysfunction has been associated with disrupted calcium signaling, oxidative stress, and amyloid-beta accumulation. Similarly, alterations in Na+/K+ pump activity have been linked to dopaminergic neuron vulnerability in Parkinson's disease. This paradigm shift offers exciting therapeutic possibilities for neurodegenerative and neuropsychiatric disorders, including schizophrenia and depression, redefining the pump's significance across multiple disciplines of medicine.
Keywords: Na+/K+ pump; Ouabain; endocrine system; hypertension; intracellular signaling.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflicts of interest or financial disclosures related to this research.
Figures

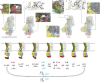

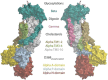

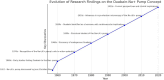

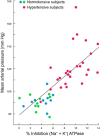


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Regulation of Na+, K+-ATPase by cardiotonic steroids: Participation in the sodium theory for migraine.Headache. 2025 Jun 18. doi: 10.1111/head.14980. Online ahead of print. Headache. 2025. PMID: 40534097 Review.
-
Mediators of the stimulatory effect of S1P on colonic Na+/K+ ATPase.PLoS One. 2025 Aug 26;20(8):e0330818. doi: 10.1371/journal.pone.0330818. eCollection 2025. PLoS One. 2025. PMID: 40857274 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- Lopina O, Bukach O, Sidorenko S, et al. Na+,K+-ATPase as a polyfunctional protein. Biochem Mosc Suppl Ser Membr Cell Biol 2022;16:207–16.
Publication types
LinkOut - more resources
Full Text Sources
