Advances in the Synthetic Approaches to β‑Secretase (BACE-1) Inhibitors in Countering Alzheimer's: A Comprehensive Review
- PMID: 40852238
- PMCID: PMC12368692
- DOI: 10.1021/acsomega.5c04467
Advances in the Synthetic Approaches to β‑Secretase (BACE-1) Inhibitors in Countering Alzheimer's: A Comprehensive Review
Abstract
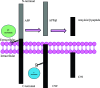
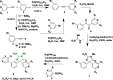
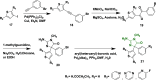
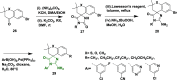
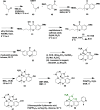
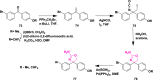
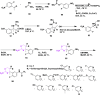
Alzheimer's disease is a progressive, irreversible, neurodegenerative disease, i.e., characterized by the presence of amyloid plaques, hyperphosphorylated tau protein (hyper p-tau), neural damage, etc. β-amyloid precursor protein cleavage enzyme 1 (BACE-1) inhibition is a promising avenue for slowing AD progression. In a rate-limiting step, BACE-1 cleaves the amyloid precursor protein (APP) into soluble amyloid precursor protein β (sAPPβ) and a membrane-bound C-terminal fragment called C99. γ-secretase processes C99, resulting in neurotoxic amyloid β (Aβ). Selective and potent BACE-1 inhibitors offer promising therapeutic avenues for Alzheimer's disease. While BACE-1 inhibitors have shown significant assurance as potential treatments for Alzheimer's disease, many early compounds struggled to advance clinically due to poor brain penetration, limited selectivity, and unwanted side effects. Over the last two decades, substantial progress has been made in the development of BACE-1 inhibitors, leading to the emergence of diverse structural frameworks such as aminohydontoins, dihydropyridines, pyrimidines, and iminohydantoins, and fused heterocycles. This review provides an in-depth analysis of the synthetic strategies employed. It emphasizes the structure-activity relationship (SAR) trends that have guided their optimization and the crystal structure of the enzyme used in the inhibition study.
© 2025 The Authors. Published by American Chemical Society.
Figures





















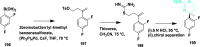
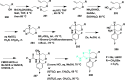
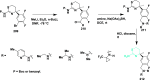








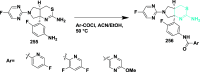




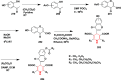































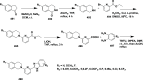




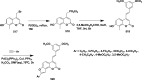
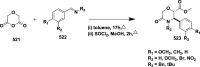



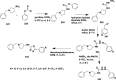
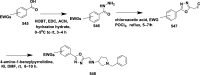

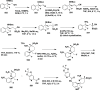
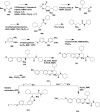
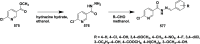

References
-
- Shah U., Shah A., Patel S., Patel A., Patel M., Solanki N., Patel S., Patel A., Patel V., Patel B.. Atorvastatin’s Reduction of Alzheimer’s Disease and Possible Alteration of Cognitive Function in Midlife as Well as Its Treatment. CNS Neurol Disord Drug Targets. 2023;22(10):1462–1471. doi: 10.2174/1871527322666221005124808. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
