Case Report: Application of ex-vivo drug sensitivity testing to identify personalized treatment options for an adolescent with diffuse midline glioma
- PMID: 40852486
- PMCID: PMC12367499
- DOI: 10.3389/fonc.2025.1606575
Case Report: Application of ex-vivo drug sensitivity testing to identify personalized treatment options for an adolescent with diffuse midline glioma
Abstract
Diffuse midline glioma (DMG) is a pediatric brain cancer that has a dismal prognosis with limited treatment options. We present the treatment course and outcome of an adolescent male diagnosed with a thalamic DMG carrying a histone H3.3 K27M (H3K27M) alteration. Tumor biopsies were taken at diagnosis for histological analysis, molecular profiling, and ex vivo drug sensitivity testing (DST). Seven months after diagnosis, the patient had recurrent/progressive disease after radiotherapy and an ineffective molecular-guided therapy based on tumor molecular profiling. The patient then started a novel functional precision medicine (FPM)-guided two-drug combination of disulfiram, based on the DST results of this drug on the patient's tumor cells obtained at diagnosis, and ONC 201, the only drug that has advanced to a phase III clinical trial for H3K27M-DMG. Neuroimaging demonstrated a treatment response, and the patient lived for fifteen months after starting this personalized therapy. Disulfiram was discontinued after three months due to significant peripheral neuropathy. Our case describes the feasibility and limitations of using DST of patient-derived tumor cells to identify potentially effective personalized and novel therapies for DMG, which should be evaluated for efficacy and safety in formal N-of-1 clinical trials settings. We discuss the benefits and risks of this approach, particularly considering its use in children, adolescents, and young adults with pediatric brain cancers.
Keywords: diffuse midline glioma; drug sensitivity testing; functional precision medicine; molecular guided therapy; pediatric brain cancer.
Copyright © 2025 Tan, Martins, Becker, Wechsler-Reya and Crawford.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
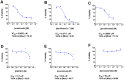
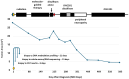
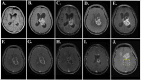
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

