Probiotic-derived extracellular vesicles: the next breakthrough in postbiotics for rheumatoid arthritis
- PMID: 40852703
- PMCID: PMC12367668
- DOI: 10.3389/fimmu.2025.1620185
Probiotic-derived extracellular vesicles: the next breakthrough in postbiotics for rheumatoid arthritis
Abstract
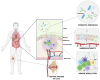
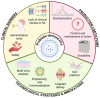
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation and joint damage. Emerging evidence highlights the role of gut and oral microbiota in RA pathogenesis, with microbial dysbiosis potentially exacerbating inflammation and immune dysregulation. Although probiotics have shown potential in modulating the oral and gut microbiota and improving RA symptoms, a promising cell-free substitute is provided by postbiotics, including probiotic-derived extracellular vesicles (EVs). These bioactive nanoparticles transport functional metabolites capable of modulating immune responses, reducing inflammation, and restoring gut barrier integrity. Probiotic-derived EVs are, for instance, able to promote M2 macrophage polarization and suppress pro-inflammatory cytokines, thus highlighting their therapeutic potential. Nonetheless, challenges remain in standardizing EVs production, optimizing administration routes, and ensuring clinical safety. The targeting and effectiveness of probiotic EVs may be improved by developments in omics sciences and biotechnology techniques, making them the next breakthrough in postbiotics for the treatment of RA. This review examines how probiotic-derived EVs interact with the host, focusing on their crosstalk with immune cells and subsequent immune modulation. We highlight their potential for RA treatment, discuss clinical challenges, and explore their use in personalized medicine.
Keywords: arthritis; dysbiosis; extracellular vesicles; immunomodulation; inflammation; oral-gut-joint axis; probiotics; therapeutic strategies.
Copyright © 2025 Dell’Atti, Abreu, Malfa, Raineri, Cappellano and Chiocchetti.
Conflict of interest statement
Author PM was employed by company SynBalance Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Immunomodulatory properties of the gut microbiome: diagnostic and therapeutic potential for rheumatoid arthritis.Clin Exp Med. 2025 Jul 1;25(1):226. doi: 10.1007/s10238-025-01777-x. Clin Exp Med. 2025. PMID: 40591032 Free PMC article. Review.
-
Microbial imbalance in the gut: a new frontier in Rheumatoid arthritis research.Inflammopharmacology. 2025 May;33(5):2277-2291. doi: 10.1007/s10787-025-01737-7. Epub 2025 Apr 12. Inflammopharmacology. 2025. PMID: 40220199 Review.
-
Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis.Mol Biol Rep. 2025 Jun 21;52(1):617. doi: 10.1007/s11033-025-10714-w. Mol Biol Rep. 2025. PMID: 40544212 Review.
-
Gut Microbiota-Immune Axis in the Regulation of Rheumatoid Arthritis: From Mechanism to Precision Probiotic Strategies.Mod Rheumatol. 2025 Aug 29:roaf081. doi: 10.1093/mr/roaf081. Online ahead of print. Mod Rheumatol. 2025. PMID: 40879288
-
DS-Modified Paeoniflorin pH-Responsive Lipid-Polymer Hybrid Nanoparticles for Targeted Macrophage Polarization in a Rat Model of Rheumatoid Arthritis.Int J Nanomedicine. 2025 Jul 12;20:8967-8992. doi: 10.2147/IJN.S516434. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40671689 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

