Photo-thermal Catalytic CO2 Methanation by RuOx@MIL-101(Cr) with 9.2% Apparent Quantum Yield under Visible Light Irradiation
- PMID: 40852772
- PMCID: PMC12412102
- DOI: 10.1021/acsami.5c10215
Photo-thermal Catalytic CO2 Methanation by RuOx@MIL-101(Cr) with 9.2% Apparent Quantum Yield under Visible Light Irradiation
Abstract
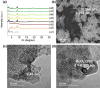
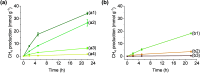
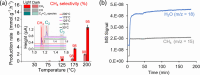
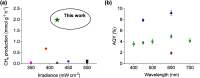
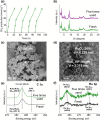
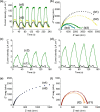
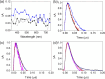
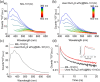
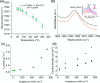
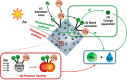
Solar-assisted gaseous CO2 hydrogenation to CH4 is a potential strategy for favoring the transition to net zero emissions. Here, we report the development of a series of efficient metal-organic frameworks with MIL-101(Cr or Fe) topology decorated with RuOx nanoparticles (ca. 0.2-2 wt %) as heterogeneous photocatalysts for the selective methanation of CO2 by H2 under simulated sunlight irradiation. The activity of RuOx(1 wt %)@MIL-101(Cr) is between 3 and 50 times higher than related MOF-based photocatalysts under similar reaction conditions. Among the different photocatalysts, the optimized RuOx(2 wt %)@MIL-101(Cr) photocatalyst showed 98.1% CO2 conversion with 98.8% CH4 selectivity reaching a production rate of 7.85 mmol g-1 h-1 with 720 mW cm-2 at 200 °C. Further, this photocatalyst exhibited a record apparent quantum yield of 9.2% at 600 nm and 200 °C after subtracting thermal activity contribution compared to any previous MOF- or other heterogeneous-based photocatalyst reported so far. The photocatalyst retained its activity and integrity upon reuse for about 110 h. Transient photocurrent, electrochemical impedance, photoluminescence, and laser flash photolysis spectroscopies together with additional photocatalytic experiments suggest the occurrance of dual photochemical and photothermal reaction pathways. The photocatalytic CO2 methanation reaction mechanism was further investigated using operando Fourier transform infrared spectroscopy.
Keywords: CO2 methanation; MIL-101(Cr); RuOx nanoparticles; metal−organic frameworks; photo-thermal catalysis.
Figures




References
-
- Bos K., Gupta J.. Stranded assets and stranded resources: Implications for climate change mitigation and global sustainable development. Energy Res. Soc. Sci. 2019;56:101215. doi: 10.1016/j.erss.2019.05.025. - DOI
-
- Gabrielli P., Rosa L., Gazzani M., Meys R., Bardow A., Mazzotti M., Sansavini G.. Net-zero emissions chemical industry in a world of limited resources. One Earth. 2023;6:682–704. doi: 10.1016/j.oneear.2023.05.006. - DOI
-
- Burkart M. D., Hazari N., Tway C. L., Zeitler E. L.. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 2019;9:7937–7956. doi: 10.1021/acscatal.9b02113. - DOI
-
- Rehman A., Nazir G., Rhee K. Y., Park S.-J.. Electrocatalytic and photocatalytic sustainable conversion of carbon dioxide to value-added chemicals: State-of-the-art progress, challenges, and future directions. J. Environ. Chem. Eng. 2022;10:108219. doi: 10.1016/j.jece.2022.108219. - DOI
LinkOut - more resources
Full Text Sources

