Impact of a Novel Dietary Supplement on Efficacy of Pharmacological Treatments for Androgenic Alopecia: A Real-Life, Multicenter, Randomized, Assessor-Blinded Trial on 225 Subjects
- PMID: 40853071
- PMCID: PMC12376952
- DOI: 10.1111/jocd.70388
Impact of a Novel Dietary Supplement on Efficacy of Pharmacological Treatments for Androgenic Alopecia: A Real-Life, Multicenter, Randomized, Assessor-Blinded Trial on 225 Subjects
Abstract
Background: Minoxidil and finasteride are currently the only FDA-approved pharmacological treatments for androgenic alopecia (AGA) and female androgenic alopecia (FAGA). However, substantial improvement is observed in no more than 20% of patients in the medium term. To enhance clinical responses, nonpharmacological dietary supplementation is often utilized.
Aims: This study evaluated the efficacy of a novel dietary supplement, AGA-P, which contains Serenoa repens extract, Cucurbita pepo extract, L-Cystine, and Vitamin C, in a multicenter, randomized, assessor-blinded, real-life trial alongside pharmacological treatments. The objective was to determine whether dietary supplementation could improve the clinical efficacy of minoxidil and finasteride.
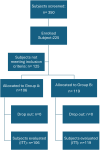
Patients and methods: A total of 225 subjects with AGA or FAGA (165 men, mean age 40 ± 14 years, range 18-74) were enrolled after obtaining informed consent. Inclusion criteria included male subjects over 18 years and postmenopausal women with mild to moderate AGA/FAGA, eligible for dietary and/or pharmacological treatment. Of these participants, 106 (24 women and 82 men) were assigned to receive pharmacological treatment plus dietary supplementation (one capsule daily; Group A), while 119 (36 women and 83 men) received drug treatment only (Group B). The pharmacological treatments consisted of topical minoxidil and oral finasteride in most cases. Treatment duration was 6 months.
Results: The results indicated that oral supplementation significantly increased the clinical efficacy of pharmacological treatments for mild-to-severe AGA/FAGA compared to drug treatment alone (great improvement: Group A 36.5% vs. Group B 25%; p = 0.04).
Conclusion: Oral supplementation of AGA-P significantly increases the clinical efficacy of pharmacological treatments for mild-to-severe AGA/FAGA (Study Registration: ISRCTN-19671217).
Keywords: Cucurbita pepo extract; Serenoa repens extract; androgenic alopecia; controlled trial.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Massimo Milani and Stefano Alfano are employees of Cantabria Labs Difa Cooper, the company selling the AGA‐P product. All other authors have no conflicts of interest.
Figures
References
-
- Lolli F., Pallotti F., Rossi A., et al., “Androgenetic Alopecia: A Review,” Endocrine 57 (2017): 9–17. - PubMed
-
- Adil A. and Godwin M., “The Effectiveness of Treatments for Androgenetic Alopecia: A Systematic Review and Meta‐Analysis,” Journal of the American Academy of Dermatology 77, no. 1 (2017): 136–141.e5. - PubMed
-
- Saraswat A. and Kumar B., “Minoxidil vs Finasteride in the Treatment of Men With Androgenetic Alopecia,” Archives of Dermatology 139, no. 9 (2003): 1219–1221. - PubMed
-
- Asnaashari S. and Javadzadeh Y., “Herbal Medicines for Treatment of Androgenic Alopecia,” Alternative Therapies in Health and Medicine 26, no. 4 (2020): 27–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous