Glucagon Receptor Deficiency Causes Early-Onset Hepatic Steatosis
- PMID: 40854221
- PMCID: PMC12451087
- DOI: 10.2337/db25-0209
Glucagon Receptor Deficiency Causes Early-Onset Hepatic Steatosis
Abstract
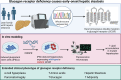
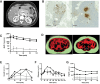
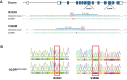
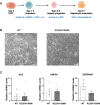
In mice, glucagon regulates lipid metabolism by activating receptors in the liver; however, its role in human lipid metabolism is incompletely understood. Here we describe three normal-weight individuals from a consanguineous family with early-onset hepatic steatosis and/or cirrhosis. Using exome sequencing, we found they were homozygous for two missense variants in the glucagon receptor gene (GCGR). In cells, the double GCGR mutation reduced cell membrane expression and signaling, resulting in an almost complete loss of function. Carriers of pathogenic GCGR mutations had substantially elevated circulating glucagon and amino acid levels and increased adiposity. Introducing the double GCGR mutation into human induced pluripotent stem cell-derived hepatocytes using CRISPR/Cas9 caused increased lipid accumulation. Our results provide an explanation for increased liver fat seen in clinical trials of GCGR antagonists and reduced liver fat in people with obesity and steatotic liver disease treated with GCGR agonists.
Article highlights: In this study, we investigated a consanguineous family in whom normal-weight individuals had hepatic steatosis and cirrhosis. Using whole-exome sequencing we found two rare homozygous variants in the glucagon receptor (GCGR) gene that cosegregated with the phenotype. In cells, the GCGR mutations result in a loss of function and increased lipid accumulation. These results highlight the potential risks associated with GCGR antagonists and the benefits of GCGR agonists, currently in clinical trials.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures


References
-
- A century of glucagon. Nat Rev Endocrinol 2023;19:311. - PubMed
-
- Sipos B, Sperveslage J, Anlauf M, et al. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab 2015;100:E783–E788 - PubMed
-
- Yu R. Mahvash disease: 10 years after discovery. Pancreas 2018;47:511–515 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

