The cat's out of the bag: Toxoplasma gondii provides further insight into myeloid-mediated host defense
- PMID: 40854593
- PMCID: PMC12377914
- DOI: 10.1093/immhor/vlaf037
The cat's out of the bag: Toxoplasma gondii provides further insight into myeloid-mediated host defense
Abstract
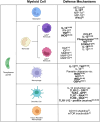
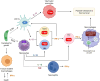
The obligate intracellular protozoan pathogen Toxoplasma gondii is estimated to infect a third of the world's population. Toxoplasmosis is considered a significant worldwide disease that can lead to morbidity or death in immunocompromised individuals. Host defense against T. gondii has been demonstrated to be dependent on a rapid myeloid cell and lymphocyte response working in concert to quickly eliminate the invading pathogen. Classically, T-bet-dependent group 1 innate lymphocytes (ILC1s), natural killer (NK) cells, and CD4+ T cell-derived interferon-γ (IFN-γ) are considered indispensable for host resistance against T. gondii. However, recent discoveries have illustrated that T-bet is not required for NK cell- or CD4+ T cell-derived IFN-γ. Yet, lack of T-bet still results in rapid mortality, pointing to a T-bet-dependent myeloid cell-mediated host defense pathway. This review summarizes the myeloid cell-mediated immune response against T. gondii and provides insights into the lesser known components of the T-bet-dependent myeloid cell-dependent host defense pathway for pathogen clearance.
Keywords: T-bet; immunoparasitology; innate immunity; myeloid cells.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
IL-12 Mediates T-bet-Expressing Myeloid Cell-Dependent Host Resistance against Toxoplasma gondii.Immunohorizons. 2024 Apr 1;8(4):355-362. doi: 10.4049/immunohorizons.2400029. Immunohorizons. 2024. PMID: 38687282 Free PMC article.
-
T-bet-dependent ILC1- and NK cell-derived IFN-γ mediates cDC1-dependent host resistance against Toxoplasma gondii.PLoS Pathog. 2021 Jan 19;17(1):e1008299. doi: 10.1371/journal.ppat.1008299. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33465134 Free PMC article.
-
Toxoplasma gondii parasites induce a localized myeloid cell immune response surrounding parasites in the brain during acute infection.mBio. 2025 Jul 9;16(7):e0081025. doi: 10.1128/mbio.00810-25. Epub 2025 Jun 10. mBio. 2025. PMID: 40492741 Free PMC article.
-
The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: a mini-review.Parasitol Res. 2021 Jul;120(7):2303-2309. doi: 10.1007/s00436-021-07204-w. Epub 2021 Jun 10. Parasitol Res. 2021. PMID: 34110502 Review.
-
A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population.Parasit Vectors. 2012 Nov 26;5:271. doi: 10.1186/1756-3305-5-271. Parasit Vectors. 2012. PMID: 23181616 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

