Efficacy and safety of one-time autologous tumor-infiltrating lymphocyte cell therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma
- PMID: 40854613
- PMCID: PMC12382571
- DOI: 10.1136/jitc-2025-011633
Efficacy and safety of one-time autologous tumor-infiltrating lymphocyte cell therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma
Abstract
Background: Recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC) has a high recurrence rate after first-line immunotherapy or chemoimmunotherapy. The presence of a high density of tumor-infiltrating lymphocytes (TILs) in HNSCC tumors was shown to be associated with improved clinical outcomes. One-time autologous TIL cell therapy was evaluated in patients with recurrent and/or metastatic HNSCC.
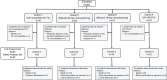
Methods: C-145-03 (NCT03083873) was a phase 2 study of TIL in patients with recurrent and/or metastatic HNSCC assigned to 1 of 4 treatment cohorts: cohort 1, non-cryopreserved TIL; cohort 2, cryopreserved lifileucel (22-day manufacturing); cohort 3, cryopreserved lifileucel (16-day manufacturing); cohort 4, cryopreserved LN-145-S1 programmed cell death protein-1 (PD-1) selected. Patients underwent tumor resection for TIL generation. After preparative non-myeloablative lymphodepletion, patients received a single infusion of TIL followed by interleukin-2 (IL-2) infusion(s). The primary endpoint was investigator-assessed objective response rate (ORR) per Response Evaluation Criteria for Solid Tumors (RECIST) V.1.1. Secondary endpoints were investigator-assessed duration of response (DOR), disease control rate (DCR), progression-free survival, overall survival, and incidence of treatment-emergent adverse events.
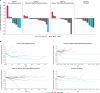
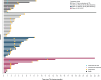
Results: Overall, 53 patients received TIL: cohort 1 (n=8), cohort 2 (n=17), cohort 3 (n=16), cohort 4 (n=12). Median age was 57 years and most patients were males (87%; 46/53) with stage IV disease (98%; 52/53). Patients had a median of two prior lines of systemic therapy; 87% (46/53) of patients had prior anti-PD-1/programmed cell death ligand-1 therapy and 72% (38/53) had prior chemotherapy. The ORR was 11% (6/53) with six patients achieving partial response (cohort 1, n=3; cohort 2, n=1; cohort 4, n=2). At median follow-up of 17.9 months, the median DOR was 7.6 months. The DCR was 76% (40/53); 64% (34/53) of patients had stable disease. The safety profile was consistent with known toxicities associated with non-myeloablative lymphodepletion and IL-2 administration.
Conclusions: This study demonstrated the feasibility of consistently generating sufficient TIL from HNSCC tumors. Results from this study suggest TIL cell therapy may serve as a potential treatment option for patients with HNSCC and support further development, including TIL cell therapy combined with immune checkpoint inhibitors or other agents or with other TIL products.
Trial registration number: NCT03083873.
Keywords: Head and Neck Cancer; Immunotherapy; Tumor infiltrating lymphocyte - TIL.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: RLF: Consulting, Advisory: Addgene, Adaptimmune, Aduro Biotech, Bicara Therapeutics, Bristol Myers Squibb, Brooklyn Immunotherapeutics LLC, Catenion, Coherus BioSciences, CureVac, CytoAgents, Eisai Europe, EMD Serono, Everest Clinical Research, F Hoffmann-La Roche, Federation Bio, Genocea Biosciences, Genmab, Hookipa Biotech GmbH, Instil Bio, Kowa Research Institute, Lifescience Dynamics, MacroGenics, MeiraGTx LLC, Merck, Merus NV, Mirati Therapeutics, Nanobiotix, Novartis Pharmaceutical, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development LP, Rakuten Medical, Regeneron, Sanofi, Seagen, SIRPant Immunotherapeutics, Vir Biotechnology, Zymeworks; Clinical Trial, Research Funding: AstraZeneca/MedImmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro; Data Safety Monitoring Board: Mirror Biologics. RSL: Consulting, Advisory: Bristol Myers Squibb, Merck, CDR-Life, Vir, AstraZeneca, RAPT, Incyte; Research Funding: Bristol Myers Squibb, Incyte, AstraZeneca; Travel, Accommodations, Expenses: Bristol Myers Squibb. CHC: Advisory Board: AVEO, Bicara Therapeutics, Exelisis, Fulgent, Seagen; Paid Consultant: Genmab, Regeneron; Research Grant (institution): Acrivon, Regeneron. AJ: Consulting: Bluedot Bio, Purple Biotech, Transimmune; Grants: NCI R01CA149456, R01DE024371, and P50CA261605; Institutional Contracts: Cantargia, Debiopharm, Genentech, Iovance, Khar Biopharma, Merck, Moderna, Pfizer, Sanofi, and SQZ for trials where he is the local PI. SML: Institutional support for clinical trials: Iovance, Lyell, Pfizer, Black Diamond. AS: Consulting: EMD Serono; Speaker: Merck. JJN: Consulting: Aadi Biosciences, ANP Technologies, AstraZeneca, BioAtla, G1 Therapeutics, Genentech, KaliVir, MindMed, Naveris, Sanofi; Research Funding: Genentech, Merck; Intellectual Property: Cansera; Stock or Stock Options: AffyImmune, Amgen, Cansera, Epic Sciences, Indee Bio, Johnson & Johnson, Novartis. JEG-O: Consulting: Aadi, Deciphera, Ipsen; Research Funding: Boehringer Ingelheim, Inhibrx, PTC therapeutics. RR: Funding to institution for clinical trial support: Coherus BioSciences, Dragonfly, Merck, Molecular Templates, RAPT Therapeutics. SJW: Invited Speaker: Merck Sharp & Dohme, Bristol Myers Squibb, Sanofi, Sun Pharma, Pierre Fabre, and AstraZeneca; Advisory Board: Bristol Myers Squibb, and Philogen; Research Grant: Novartis; Consulting/Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Sanofi, and Sun Pharma; Travel, Accommodations, Expenses: Pierre Fabre and Sun Pharma. VMV: Advisory: AstraZeneca, Coherus BioSciences, Regeneron; Research Support: Takeda. JM: Nothing to disclose. FGF: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics; Patents, Royalties, Other Intellectual Properties: Bristol Myers Squibb. JC: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics. BG: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics. RF: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics. MC: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics. MY: Employment: Iovance Biotherapeutics; Stock or Stock Options: Iovance Biotherapeutics; Travel, Accommodations, Expenses: Iovance Biotherapeutics. EEWC: Employment: Tempus AI.
Figures
References
-
- Rahway, NJ, USA: Merck & Co., Inc; 2024. Keytruda [package insert]
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
