Who truly benefits from gilteritinib combinations in FLT3-mutated relapsed-refractory(R/R) AML: a Canadian single center analysis
- PMID: 40854875
- PMCID: PMC12378385
- DOI: 10.1038/s41408-025-01353-2
Who truly benefits from gilteritinib combinations in FLT3-mutated relapsed-refractory(R/R) AML: a Canadian single center analysis
Abstract
Conflict of interest statement
Competing interests: This study has not been funded by any pharmaceutical companies. There were no direct COI specifically related to this research project. ADS has received research funding from Takeda Pharmaceuticals, BMS and Medivir AB, and consulting fees/honorarium from Takeda, Novartis, Jazz, and Otsuka Pharmaceuticals. ADS is named on a patent application for the use of DNT cells to treat AML. ADS is a member of the Medical and Scientific Advisory Board of the Leukemia and Lymphoma Society of Canada. The remaining authors declare no competing interests. Ethics approval statement: The study was approved by research ethics board (REB) and waiver of consent was granted. Patient consent statement: Waiver of consent was granted by the REB.
Figures
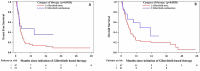
References
-
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

