Bone marrow microenvironment in autoimmune hemolytic anemia: from trephine biopsy to single cell RNA sequencing
- PMID: 40854885
- PMCID: PMC12379653
- DOI: 10.1038/s41392-025-02348-y
Bone marrow microenvironment in autoimmune hemolytic anemia: from trephine biopsy to single cell RNA sequencing
Abstract
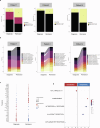
The role of bone marrow (BM) compensatory response in autoimmune hemolytic anemias (AIHAs) is emerging and inadequate reticulocytosis has been associated with more severe disease and adverse outcomes. However, few is known about the BM immunologic microenvironment composition in these diseases. Here we investigated BM features in a large cohort of 97 patients with autoimmune hemolytic anemia (AIHA) and observed a high prevalence of hypercellularity, dyserythropoiesis, reticulin fibrosis, and T-cell infiltration (65%, 29%, 76%, and 69% of patients, respectively). These findings were associated with inadequate bone marrow compensation, more severe anemia at onset, and need of multiple treatments. In a subset of warm type AIHA patients we investigated BM microenvironment by single-cell RNA sequencing. We found distinct immune cell profiles across disease stages (diagnosis, remission, relapse). In particular, upregulation of inflammatory response pathways was noted in CD8 + , CD4 + , and monocyte subsets during relapse compared to diagnosis and remission. Moreover, by single-cell TCR sequencing, we found small T cell clones at diagnosis that may either disappeared or expanded at remission. Disappearing clones exhibited a naive CD8+ phenotype and were more likely to respond to glucocorticoid treatment. Expanding clones showed upregulation of cytotoxic T cell markers and may play a role in the transition to a chronic/relapsing phase. Finally, cytokine gene expression differed across disease phases. At relapse, pro-inflammatory cytokines such as TNF-alpha, IL-1, and IL-6 were upregulated in CD4+ and CD8 + T cells, while TGF-beta was downregulated, potentially in an attempt to counteract the transition to chronic phase. This is the largest study evaluating BM histology and clinical characteristics, and the first evaluation of BM microenvironment by single-cell RNA sequencing in AIHA. We showed a complex scenario encompassing T-cell infiltration, clonality, and up/down-regulation of cytokine genes, associated with a more severe and relapsing disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Petz, L. D., Garratty G. (eds) Immune Hemolytic Anemias (Churchill Livingstone Press, 2003).
-
- Jäger, U. et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev.41, 100648 (2020). - PubMed
-
- Berentsen, S. & Barcellini, W. Autoimmune hemolytic anemias. N. Engl. J. Med.385, 1407–1419 (2021). - PubMed
-
- Barcellini, W. & Fattizzo, B. How I treat warm autoimmune hemolytic anemia. Blood141, 438–439 (2023). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

