Impact of in vitro exposure to 5G-modulated 3.5 GHz fields on oxidative stress and DNA repair in skin cells
- PMID: 40854925
- PMCID: PMC12379245
- DOI: 10.1038/s41598-025-15090-w
Impact of in vitro exposure to 5G-modulated 3.5 GHz fields on oxidative stress and DNA repair in skin cells
Abstract
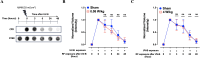
The rapid deployment of fifth-generation (5G) wireless networks has raised societal concerns regarding potential biological effects, particularly on human skin, due to the use of higher carrier frequencies that penetrate tissue less deeply. Consequently, whether 5G-modulated radiofrequency (RF) electromagnetic fields (EMFs) at 3.5 GHz affect oxidative stress and DNA repair in skin cells remains an open question. Using genetically encoded Bioluminescence Resonance Energy Transfer (BRET)-based biosensors targeted to the cytoplasm and mitochondria, we assessed whether exposure of human fibroblasts to 5G RF-EMF at specific absorption rates (SAR) of 0.08 and 4 W/kg for 24 h could alter basal reactive oxygen species (ROS) levels or potentiate the effects of known ROS inducers, including H₂O₂, Kp372-1, and Antimycin A. We also evaluated whether pre-exposure to 5G RF-EMF could induce an adaptive response (AR), by modulating ROS production following a subsequent challenge with arsenic trioxide (As₂O₃). Additionally, we investigated the impact of combined RF-EMF and ultraviolet-B (UV-B) exposure on the formation and repair of cyclobutane pyrimidine dimer (CPD) lesions in HaCaT keratinocytes. Our results showed no significant effect of 5G RF-EMF exposure, either alone or in combination with chemical ROS inducers, on oxidative stress markers in either compartment. Likewise, RF-EMF exposure did not induce an adaptive response to oxidative challenge, nor did it alter the kinetics or the efficiency of CPD repair by the nucleotide excision repair (NER) pathway. These findings support the conclusion that the exposure to 5G RF-EMF at 3.5 GHz up to 4 W/kg does not induce oxidative stress or impair DNA repair efficiency in human skin cells, within the experimental conditions tested.
Keywords: Bioluminescence resonance energy transfer (BRET); Cellular stress response; Cyclobutane pyrimidine dimer (CPD); DNA damage; Fifth generation (5G); Nucleotide excision repair (NER); Oxidative stress; Radiofrequency electromagnetic fields (RF-EMF); Reactive oxygen species (ROS).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

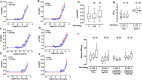
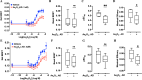
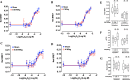
References
-
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 118, 483–524 (2020). - PubMed
-
- Baan, R. et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol.12, 624–626 (2011). - PubMed
-
- Gopal, B. G. A comparative study on 4G and 5G technology for wireless applications. IOSR J. Electron. Commun. Eng. IOSR-JECE10(6), 2278–2834 (2015).
-
- Mahmud, M. Cellular mobile technologies (1G to 5G) and massive MIMO. Int. J. Sci. Res. IJSR8, 929–937 (2019).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

