Circulating galectin-9 as a novel prognosticator in patients with intrahepatic cholangiocarcinoma undergoing surgical resection
- PMID: 40854937
- PMCID: PMC12378371
- DOI: 10.1038/s41598-025-15310-3
Circulating galectin-9 as a novel prognosticator in patients with intrahepatic cholangiocarcinoma undergoing surgical resection
Abstract
Background: Intrahepatic cholangiocarcinoma (ICC) is a rare but highly malignant liver cancer. Surgical resection provides the best long-term survival, yet poor prognosis requires improved treatments. Galectin-9 (GAL9) has gained attention for its role in tumor biology. This study investigated circulating galectin-9 (cGAL9) levels in ICC patients undergoing resection, along with tumor tissue characteristics.
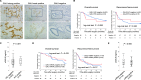
Methods: GAL9 expression levels in circulating and tumor cells were measured using enzyme-linked immunosorbent assay and immunohistochemistry (IHC) in 91 ICC surgical patients. The mRNA expression levels of candidate genes were analyzed in 44 available frozen tissue samples.
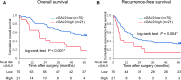
Results: The optimal cGAL9 cutoff was 12.0 ng/ml using minimum P value approach. Higher cGAL9 levels linked to multiple tumors (P = 0.046), poorer overall survival (OS), and recurrence-free survival (RFS). Tumor cell GAL9 expression by IHC did not correlate with OS or RFS. cGAL9 levels did not correlate with tumor cell expression in IHC analyses or GAL9 mRNA in resected specimens. However, cGAL9 levels correlated with mRNA levels of glycolysis markers (glucose transporter 1 and hypoxia inducible factor 1 alpha).
Conclusions: Preoperative cGAL9 serves as a novel prognosticator for ICC patients after surgical resection. Its association with glycolysis highlights the potential for therapeutic guidance.
Keywords: Galectin 9; Glut1; Hif 1 alpha; Intrahepatic cholangiocarcinoma; Liver cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Opt-out informed consent was obtained to use the participant data for research. Informed consent was obtained from all participants and/or their legal guardians. This study was approved by the Ethics Committee of Kyoto University Hospital, Graduate School and Faculty of Medicine (Approval No. R3844), and performed in accordance with the Declaration of Helsinki. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

