Antibody dependent complement activation is critical for boosting opsonophagocytosis of Staphylococcus epidermidis in an extremely preterm human whole blood model
- PMID: 40855249
- PMCID: PMC12378347
- DOI: 10.1038/s41598-025-15490-y
Antibody dependent complement activation is critical for boosting opsonophagocytosis of Staphylococcus epidermidis in an extremely preterm human whole blood model
Abstract
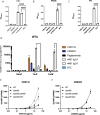
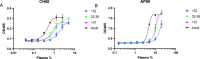
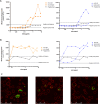
Staphylococcus epidermidis is a major cause of late onset sepsis in extremely preterm neonates. Antibody therapies are considered as an interesting strategy to prevent sepsis. However, previous clinical trials with intravenous immunoglobulin (IVIG) and a monoclonal antibody (mAb) targeting staphylococcal lipoteichoic acid (LTA) (Pagibaximab) failed to show significant protection from invasive infections in extremely preterm neonates. Here we use an age-specific in vitro platform to compare immune protection by Pagibaximab with two other mAbs recognizing invasive S. epidermidis in the context of the neonatal immune system. We demonstrate poor activity of Pagibaximab in inducing complement C3b opsonization and neutrophil opsonophagocytosis in neonatal plasma. MAbs CR5133 and CR6453 [recognizing wall teichoic acid (WTA)] potently induced S. epidermidis opsonophagocytosis in an (extreme) preterm reconstituted whole blood model, especially after introduction of hexamer-enhancing mutations in the IgG-Fc tail. In conclusion, using age-specific in vitro assays, we show that mAbs with strong complement-inducing potential may be effective in preventing neonatal bacterial sepsis in extreme preterm neonates.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Monoclonal antibodies effectively potentiate complement activation and phagocytosis of Staphylococcus epidermidis in neonatal human plasma.Front Immunol. 2022 Jul 29;13:933251. doi: 10.3389/fimmu.2022.933251. eCollection 2022. Front Immunol. 2022. PMID: 35967335 Free PMC article.
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2013 Jul 02;(7):CD000361. doi: 10.1002/14651858.CD000361.pub3. PMID: 14973955 Updated.
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2001;(2):CD000361. doi: 10.1002/14651858.CD000361. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. PMID: 11405962 Updated.
-
Novel broadly reactive monoclonal antibody protects against Pseudomonas aeruginosa infection.Infect Immun. 2025 Jan 31;93(1):e0033024. doi: 10.1128/iai.00330-24. Epub 2024 Dec 13. Infect Immun. 2025. PMID: 39670709 Free PMC article.
-
Intravenous immunoglobulin for suspected or subsequently proven infection in neonates.Cochrane Database Syst Rev. 2004;(1):CD001239. doi: 10.1002/14651858.CD001239.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2010 Mar 17;(3):CD001239. doi: 10.1002/14651858.CD001239.pub3. PMID: 14973965 Updated.
References
-
- Cailes, B. et al. Epidemiology of UK neonatal infections: The neonIN infection surveillance network. Arch. Dis. Child. Fetal Neonatal. Ed.103, F547–F553 (2018). - PubMed
-
- Selvaraj, P., Fifadara, N., Nagarajan, S., Cimino, A. & Wang, G. Functional regulation of human neutrophil Fc gamma receptors. Immunol. Res.29, 219–230 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

