Sodium-glucose cotransporter 2 inhibitors alleviate renal fibrosis in diabetic kidney disease by inhibiting Hmgcs2 and Btg2 in proximal tubular cells
- PMID: 40855324
- PMCID: PMC12379496
- DOI: 10.1186/s12967-025-06788-6
Sodium-glucose cotransporter 2 inhibitors alleviate renal fibrosis in diabetic kidney disease by inhibiting Hmgcs2 and Btg2 in proximal tubular cells
Abstract
Context: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been shown to ameliorate renal fibrosis in diabetic kidney disease (DKD), but the mechanism has not been fully explored.
Methods: The single-cell sequencing (scRNA-seq) data were downloaded from the Gene Expression Omnibus (GEO) database, and we selected the tissue data from db/m mice, db/db mice and db/db mice with SGLT2i treatment. The results were also validated by immunofluorescent staining and western blot in vivo and in vitro, respectively.
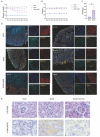
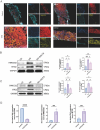
Results: Our study demonstrates that SGLT2i directly ameliorated fibrosis of proximal tubular cells by downregulating 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2) expression in S1 proximal tubular segment cells (PT_S1) and decreasing the number of proximal tubular cell cluster with B-cell translocation gene 2 (Btg2) highly expressed (Btg2_PT). In addition, SGLT2i could indirectly influence macrophages through cell-cell communication between epithelial cells and macrophages, specifically via the App-CD74 ligand-receptor pair, thus suppressing the inflammatory response in macrophages, ultimately contributing to the delay in DKD progression.
Conclusion: Our study found that Hmgcs2 and Btg2 are therapeutic targets for Sodium-glucose cotransporter 2 inhibitors to ameliorate kidney fibrosis in diabetic kidney disease. Single-cell sequencing technology provided a high resolution of this study at the cellular level.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12967-025-06788-6.
Keywords: Btg2; Diabetic kidney disease; Hmgcs2; Renal fibrosis; SGLT2i; Single-cell sequencing.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal study and human renal samples were reviewed and approved by ZhuJiang Hospital of Southern Medical University Laboratory Ethics Committee. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
ASK1 limits kidney glucose reabsorption, growth, and mid-late proximal tubule KIM-1 induction when diabetes and Western diet are combined with SGLT2 inhibition.Am J Physiol Renal Physiol. 2025 May 1;328(5):F662-F675. doi: 10.1152/ajprenal.00031.2025. Epub 2025 Mar 28. Am J Physiol Renal Physiol. 2025. PMID: 40152436 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors ameliorate glutathione cysteine ligase modifier-mediated oxidative stress and subsequent ferroptosis in proximal tubules of diabetic kidney disease.Redox Rep. 2025 Dec;30(1):2528334. doi: 10.1080/13510002.2025.2528334. Epub 2025 Jul 28. Redox Rep. 2025. PMID: 40722167 Free PMC article.
-
Sodium-glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms.Pharmacol Rep. 2023 Feb;75(1):44-68. doi: 10.1007/s43440-022-00442-4. Epub 2022 Dec 19. Pharmacol Rep. 2023. PMID: 36534320 Review.
-
Gene deletion of the Na+-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion.Am J Physiol Renal Physiol. 2019 Jun 1;316(6):F1201-F1210. doi: 10.1152/ajprenal.00111.2019. Epub 2019 Apr 17. Am J Physiol Renal Physiol. 2019. PMID: 30995111 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors: a novel approach to prevent the transition from acute kidney injury to chronic kidney disease.Curr Opin Nephrol Hypertens. 2025 Sep 1;34(5):433-439. doi: 10.1097/MNH.0000000000001080. Epub 2025 Apr 23. Curr Opin Nephrol Hypertens. 2025. PMID: 40265513 Review.
References
-
- Tuttle KR, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102:248–60. - PubMed
-
- Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. - PubMed
-
- Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: A review. Am J Kidney Dis. 2018;72:267–77. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

