Uropathogenic Escherichia coli (UPEC) that hides its identity: features of LC2 and EC73 strains from recurrent urinary tract infections
- PMID: 40855470
- PMCID: PMC12376328
- DOI: 10.1186/s12866-025-04287-8
Uropathogenic Escherichia coli (UPEC) that hides its identity: features of LC2 and EC73 strains from recurrent urinary tract infections
Abstract
Background: Uropathogenic Escherichia coli (UPEC) strains are the major causative agents of human urinary tract infections (UTIs). Many patients who develop UTIs will experience a recurrent UTI (RUTI) within 6 months despite antibiotic-mediated clearance of the initial infection. A significant proportion of RUTIs are caused by E. coli identical to the original strain. UPEC employs several strategies to adhere, colonize, and persist within the bladder niche. Knowledge about the mechanisms regulating specific host-pathogen interactions that promote bacterial persistence is necessary to develop new approaches to RUTI diagnosis and treatment.
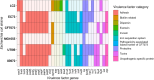
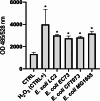
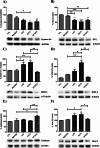
Results: LC2 and EC73 UPEC strains were collected from patients with RUTIs. E. coli CFT073 and K-12 MG1655 were used as reference strains. UPEC displayed phenotypic profiles like those of the general E. coli population. The pan-genome analysis revealed that LC2 harbored many unique genes encoding several different functions such as intracellular trafficking and secretion, and vesicular transport. Contrarily, EC73 was the strain with the lowest number of unique genes involved in replication, recombination, repair and cell wall/membrane/envelope biogenesis. LC2 and EC73 exhibited the capacity to invade bladder monolayers efficiently and to colonize the gut of Caenorhabditis elegans, with LC2 being significantly more virulent than EC73. T24 cells infected with EC73 and LC2 strains exhibited significantly increased mRNA levels of IL-6, IL-8, IL-1β and TNF-α. EC73 elicited the strongest cytokine response. Differently, no significant cytokine mRNA induction was detected in T24 cells infected with E. coli CFT073. LC2 and EC73 modulated the expression of proteins involved in reactive oxygen species (ROS) balance in infected cells, but to different extents.
Conclusion: The acquisition of virulence factors by horizontal transfer of accessory DNA, other than being the cause of transformation to pathogenic strains, is responsible for the genomic plasticity. Our findings suggest that a key role in RUTIs could be played by certain bacterial strains that may benefit from peculiar abilities to adapt and potentially develop reservoirs of persistence across different host environments.
Keywords: Bladder; Recurrence; Urinary tract infections; Uropathogenic Escherichia coli.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The bacterial strains were collected precisely in 2006 and were present in our Laboratory as collection isolates. At “Sapienza” University of Rome the Ethics Committee for Transdisciplinary Research (CERT in Italian), that ensures that Sapienza University research follows ethical principles defined by international and national regulations and the Sapienza Statute and Code of Ethics, it has been established in 2021 (RD no. 59099/2021) (se https://www.uniroma1.it/en/pagina/ethics-committee-transdisciplinary-research ). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

