History of pre-eclampsia negatively impacts stroke severity postpartum in rats
- PMID: 40855879
- PMCID: PMC12377490
- DOI: 10.1002/nep3.70002
History of pre-eclampsia negatively impacts stroke severity postpartum in rats
Abstract
Background: Preeclampsia (PE) is a serious hypertensive disorder of pregnancy that has lifelong deleterious effects, including increased risk of stroke postpartum (PP). Here we determined if previous PE exacerbates ischemic injury in the PP period and investigated underlying mechanisms including oxidative stress and collateral perfusion.
Methods: Female Sprague-Dawley rats were studied at 4-9 months PP, after either a normal pregnancy (NormP-PP n = 7) or experimental PE (ePE) induced via high cholesterol diet during gestation (ePE-PP n = 9). Animals underwent transient middle cerebral artery occlusion (tMCAO) for 2 hours with 1 hour reperfusion. Dual-site laser Doppler flowmetry measured changes in cerebral blood flow (CBF) in the MCA and collateral territories. Ischemic injury was measured by 2,3,5-triphenyl tetrazolium chloride staining. Circulating 8-isoprostane, 3-nitrotyrosine (3-NT), and oxidized low-density lipoprotein (oxLDL) were measured by enzyme-linked immunosorbent assays. In separate groups of animals, NormP-PP (n = 10) and ePE-PP (n = 9) that were 3-4 months PP, isolated pial collateral vessels, leptomeningeal anastomoses (LMAs), and mesenteric arteries were studied using pressure myography.
Results: Previous ePE pregnancy worsened stroke outcome in the PP state, significantly increasing infarction in ePE-PP vs. NormP-PP animals (40.6 ± 7.6% vs. 13.7 ± 6.5%; p <0.01) and edema (5.1 ± 2.0% vs. 2.6 ± 0.4%; p < 0.01), despite comparable changes in CBF in both MCA and pial collateral territories during ischemia and reperfusion. When infarction was analyzed as a function of perfusion deficit, ePE-PP animals had greater sensitivity to ischemia. Pial collaterals had increased pressure-induced myogenic tone vs. NormP-PP rats. Percent tone at 80 mmHg for ePE-PP vs. NormP-PP was 15.5 ± 1.6% vs. 8.6 ± 1.9% (p <0.01). In addition, ePE-PP animals had significantly elevated circulating 8-isoprostane and 3-NT, but not oxLDL, after tMCAO (*p<0.05 and **p<0.01, respectively).
Conclusions: We found worsened stroke outcome after ePE pregnancy that was related to increased sensitivity to ischemia, increased pial collateral tone, and elevated levels of oxidative stress markers. Thus, the pathologic effects of ePE persisted PP and negatively impacted stroke outcome.
Keywords: collateral flow; ischemic stroke; oxidative stress; postpartum; preeclampsia.
Conflict of interest statement
Conflict of Interests The authors have no relevant financial or non-financial disclosures to report. All authors declare no conflict of interest.
Figures



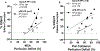
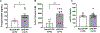

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
