Impact of dry heat sterilization on the mechanical and functional performance of 3D printed medical devices for image-guided intervention
- PMID: 40855887
- PMCID: PMC12376825
- DOI: 10.1016/j.stlm.2025.100216
Impact of dry heat sterilization on the mechanical and functional performance of 3D printed medical devices for image-guided intervention
Abstract
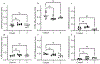
Polymer additive manufacturing (AM) is a powerful method for medical device prototyping, producing low-cost medical devices for resource limited settings and patient-specific customization. Plastic medical devices created with AM must undergo sterilization prior to in vivo experiments or use with patients. While prior studies have verified the feasibility and safety of sterilization of such devices, the impact on mechanical performance has not been studied. Temperatures during autoclave sterilization, a commonly used and widely available method, can match or exceed the melt temperature of Nylon-12 used for selective laser sintering. Here we tested the impact of single or multiple cycles of autoclave sterilization on the mechanical and functional performance of plastic components used with interventional radiology related medical devices (catheter hub, ablation probe handle, and implantable port). We found that up to 2 cycles of sterilization did not tangibly impact the manufacturability, function or surface finish of these devices. However, we found that more than one cycle of sterilization can compromise the mechanical strength of the devices, with geometric-linked variations in the level of change in stiffness (ranging from 12 - 23 %). In conclusion, autoclave sterilization is safe for single use AM medical devices, where repetition of the sterilization for device reuse can compromise mechanical performance.
Keywords: 3D printing; Additive Manufacturing; Interventional Radiology; Medical Devices; Rapid Prototyping.
Figures





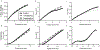
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Is Your Surgical Helmet System Compromising the Sterile Field? A Systematic Review of Contamination Risks and Preventive Measures in Total Joint Arthroplasty.Clin Orthop Relat Res. 2025 Jun 1;483(6):972-990. doi: 10.1097/CORR.0000000000003383. Epub 2025 Feb 5. Clin Orthop Relat Res. 2025. PMID: 39915114
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Device-modified trabeculectomy for glaucoma.Cochrane Database Syst Rev. 2015 Dec 1;2015(12):CD010472. doi: 10.1002/14651858.CD010472.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Mar 13;3:CD010472. doi: 10.1002/14651858.CD010472.pub3. PMID: 26625212 Free PMC article. Updated.
References
-
- da Silva LRR, Sales WF, dos AR. Campos, de Sousa JAG, Davis R, Singh A, Coelho RT, Borgohain B. A comprehensive review on additive manufacturing of medical devices. Progress in additive manufacturing, 6. Springer Science and Business Media Deutschland GmbH; 2021. p. 517–53. 10.1007/s40964-021-00188-0. Aug. 01. - DOI
-
- Fogarasi M, Snodderly KL, Di Prima MA. A survey of additive manufacturing trends for FDA-cleared medical devices. Nat Rev Bioeng 2023;1(10):687–9. 10.1038/s44222-023-00109-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
