Multi-modal comparative phenotyping of knock-in mouse models of frontotemporal dementia/amyotrophic lateral sclerosis
- PMID: 40856010
- PMCID: PMC12421800
- DOI: 10.1242/dmm.052324
Multi-modal comparative phenotyping of knock-in mouse models of frontotemporal dementia/amyotrophic lateral sclerosis
Abstract
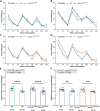
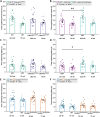
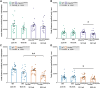
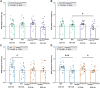
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are progressive adult-onset neurodegenerative diseases with overlapping pathological and genetic origins. They are caused by multiple underlying mechanisms leading to a common collection of clinical features that occur in a spectrum. Here, we report side-by-side longitudinal behavioural, cognitive and sensory phenotyping of two mouse models of ALS/FTD, to determine which aspects of the disease they recapitulate. We used knock-in models, in which the endogenous mouse orthologues of the C9orf72 and TARDBP (encoding TDP-43) genes have been altered to model specific molecular aspects of ALS/FTD. We found that the C9orf72GR400/+ model exhibits age-related deficit in short-term memory and that parental genotype affects exploration activity in offspring. In the TardbpQ331K/Q331K model, we found age-related changes in weight, fat mass, locomotion and marble burying. In both models, we found no evidence of deficits in vision or olfactory habituation-dishabituation. These data provide new insight into genotype-phenotype relationships in these ALS/FTD mice, which can be used to inform model choice and experimental design in future research studies.
Keywords: Amyotrophic lateral sclerosis; Frontotemporal dementia; Mouse phenotyping.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests F.K.W. has undertaken fee consultancy for Alnylam Pharmaceuticals for work unconnected to this study. The other authors declare no competing or financial interests.
Figures




References
-
- Ahmed, R. M., Irish, M., Piguet, O., Halliday, G. M., Ittner, L. M., Farooqi, S., Hodges, J. R. and Kiernan, M. C. (2016a). Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol. 15, 332-342. 10.1016/S1474-4422(15)00380-4 - DOI - PubMed
-
- Ahmed, R. M., Caga, J., Devenney, E., Hsieh, S., Bartley, L., Highton-Williamson, E., Ramsey, E., Zoing, M., Halliday, G. M., Piguet, O.et al. (2016c). Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J. Neurol. 263, 1593-1603. 10.1007/s00415-016-8168-2 - DOI - PubMed
-
- Ahmed, R. M., Irish, M., Van Eersel, J., Ittner, A., Ke, Y. D., Volkerling, A., Van Der Hoven, J., Tanaka, K., Karl, T., Kassiou, M.et al. (2017). Mouse models of frontotemporal dementia: a comparison of phenotypes with clinical symptomatology. Neurosci. Biobehav. Rev. 74, 126-138. 10.1016/j.neubiorev.2017.01.004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

