Durability of Response to B-Cell Maturation Antigen-Directed mRNA Cell Therapy in Myasthenia Gravis
- PMID: 40856088
- PMCID: PMC12623823
- DOI: 10.1002/acn3.70167
Durability of Response to B-Cell Maturation Antigen-Directed mRNA Cell Therapy in Myasthenia Gravis
Abstract
Objective: We report the 12-month follow-up outcomes from a Phase 2 clinical trial (NCT04146051) evaluating Descartes-08, a BCMA-directed RNA chimeric antigen receptor T-cell (rCAR-T) therapy for refractory generalized myasthenia gravis (MG). These findings provide insight into the potential applicability of BCMA-targeted rCAR-T therapy for antibody-mediated autoimmune diseases.
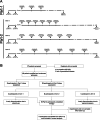
Methods: In the Phase 2a part of the study, Descartes-08 was administered at 52.5 × 106 CAR+ cells/kg per infusion with varying dosing frequencies as an outpatient treatment and without lymphodepletion chemotherapy. A subset of participants received Descartes-08 as six weekly infusions and were followed long term with assessments conducted at 2, 3, 6, 9, and 12 months.
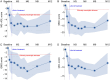
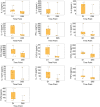
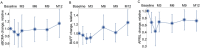
Results: All seven participants who received six weekly infusions of Descartes-08 exhibited clinically meaningful improvement in common MG severity scales (MG Composite, MG Activities of Daily Living, Quantitative MG scores, and Quality of Life 15-revised) at Month 3 without significant toxicity. At Month 9 follow-up, all participants continued to experience marked clinically meaningful improvements. Five out of seven participants maintained the response at Month 12. A third participant experienced a relapse approximately 6 months after completing on-study follow-up. All three participants who experienced loss of clinical effects were retreated. Two had rapid improvement in clinical scores with minimal symptom expression at Week 8, which was maintained through 12 months of retreatment follow-up. The third participant experienced similar improvement in MG severity scores to their initial treatment.
Interpretation: These data support continued development of Descartes-08 in myasthenia gravis and other autoantibody-associated autoimmune disorders.
Keywords: BCMA; CAR‐T; Descartes‐08; autoimmune; myasthenia gravis.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nizar Chahin has received honoraria as a consultant or advisory board member from argenx, Sanofi, Amicus, and Sarepta. Gregory Sahagian: consulting fees from UCB Biosciences, Immunovant; honoraria from argenx, Alexion; travel support from argenx, Immunovant. Marc H. Feinberg: honoraria from argenx. Christopher M. Jewell: equity in Barinthus Biotherapeutics and Nodal Therapeutics; employee: the University of Maryland and VA Maryland Health Care System. The views in this paper do not reflect the views of the state of Maryland or the US Government. C. Andrew Stewart, Christopher M. Jewell, Metin Kurtoglu, and Miloš D. Miljković: employees, ownership interest in Cartesian Therapeutics. Tuan Vu: honoraria from Alexion, argenx, Dianthus, ImmunAbs, Johnson & Johnson, NMD Pharma, and UCB Biosciences. Tahseen Mozaffar: consulting fees from Alexion Pharmaceuticals Inc., Amicus, Annji, argenx, Audentes/Astellas Gene Therapy, Horizon Therapeutics, Maze Therapeutics, Momenta, Sanofi, UCB; support for attending meetings and/or travel from Sanofi; participation on a DSMB or an advisory board from Sarepta, Applied Therapeutics, and the National Institutes of Health. James F. Howard Jr.: consulting fees from Alexion AstraZeneca Rare Disease, Amgen, argenx, Biohaven Ltd., F. Hoffmann‐LaRoche Ltd., Hansa Biopharma, Merck EMB Serono, NMD Pharma, Novartis Pharma, Regeneron Pharmaceuticals Inc., Sanofi US, Seismic Therapeutics, UCB Biosciences. All other authors declare no competing interests.
Figures


References
-
- Gilhus N. E., “Myasthenia Gravis,” New England Journal of Medicine 375, no. 26 (2016): 2570–2581. - PubMed
-
- Fujii Y., Monden Y., Hashimoto J., Nakahara K., and Kawashima Y., “Acetylcholine Receptor Antibody Production by Bone Marrow Cells in a Patient With Myasthenia Gravis,” Neurology 35, no. 4 (1985): 577–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

