Effect of Immunotherapy on Late Elderly Patients With Unresectable Hepatocellular Carcinoma: A Real-World Clinical Study
- PMID: 40856425
- PMCID: PMC12378699
- DOI: 10.1002/cam4.71171
Effect of Immunotherapy on Late Elderly Patients With Unresectable Hepatocellular Carcinoma: A Real-World Clinical Study
Abstract
Background/aim: The global aging population includes an increasing number of elderly patients with hepatocellular carcinoma (HCC). This study aimed to clarify the real-world outcomes, prognostic factors, and appropriate administration indicators for immunotherapy in elderly HCC patients.
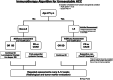
Methods: This retrospective multicenter study analyzed 286 patients with unresectable HCC who received first-line immunotherapy (atezolizumab-bevacizumab or durvalumab-tremelimumab) between November 2020 and January 2024. Patients were categorized into the late elderly (LE; ≥ 75 years, n = 117) and non-late elderly (non-LE; ≤ 74 years, n = 169) groups. Baseline characteristics, overall survival (OS), progression-free survival (PFS), and prognostic factors were evaluated.
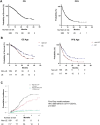
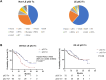
Results: The LE group had significantly poorer performance status, lower albumin-bilirubin (ALBI) scores, lower alpha-fetoprotein (AFP) and alanine transaminase levels, higher creatinine levels, and were significantly less likely to receive post-immune checkpoint inhibitor (ICI) treatment compared with the non-LE group (56.2% vs. 38.4%, p = 0.0038). Median OS and PFS for the LE group were 25.6 and 10.5 months, respectively. The LE group demonstrated a comparable disease control rate (82.0%) and safety profile. The ALBI score was a significant prognostic factor for both groups. Post-ICI treatment significantly improved OS only in the non-LE group, even after propensity score matching for ALBI score and AFP levels.
Conclusions: Immunotherapy is effective and well-tolerated in LE patients with unresectable HCC, particularly in those with preserved liver function (mALBI grade 1/2a). Post-ICI treatment significantly benefits non-LE patients, with limited impact on LE patients, highlighting the need for therapeutic strategies based on age and liver function.
Keywords: ALBI score; atezolizumab plus bevacizumab; durvalumab‐tremelimumab; hepatocellular carcinoma; post‐ICI treatment.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- WHO , “Aging and Health,” (2022), accessed December 29, 2024, https://www.who.int/news‐room/fact‐sheets/detail/ageing‐and‐health.
-
- Llovet J. M., Kelley R. K., Villanueva A., et al., “Hepatocellular Carcinoma,” Nature Reviews Disease Primers 7 (2021): 6. - PubMed
-
- Villanueva A., “Hepatocellular Carcinoma,” New England Journal of Medicine 11, no. 380 (2019): 1450–1462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

