Effects of Treadmill Exercise on Retinal Vascular Morphology, Function, and Circulating Immune Factors in a Mouse Model of Retinal Degeneration
- PMID: 40856647
- PMCID: PMC12395787
- DOI: 10.1167/iovs.66.11.65
Effects of Treadmill Exercise on Retinal Vascular Morphology, Function, and Circulating Immune Factors in a Mouse Model of Retinal Degeneration
Abstract
Purpose: Exercise is neuroprotective in rodents undergoing retinal degeneration (RD). However, the effects of exercise on retinal vasculature remain unexplored. Here, we investigate whether treadmill exercise influences retinal vascular morphology, function, gene expression, and circulating factors in a light-induced retinal degeneration (LIRD) mouse model.
Methods: Six-week-old female BALB/c mice were assigned to inactive + dim, active + dim, inactive + LIRD, and active + LIRD groups (n = 20 per group). Active mice were treadmill exercised (1 hour/day, 10 meters per minute ]m/min]) for 3 weeks, then, LIRD was induced (5000 lux/4 hours). Inactive mice were placed on a static treadmill. Five days post-LIRD, optical coherence tomography angiography (OCT-A) and functional hyperemia (FH) were performed to assess retinal vascular morphology and function in vivo, respectively. Endothelial cells were isolated from whole retina and gene expression was quantified using droplet digital PCR (ddPCR). Serum was collected for circulating cytokine and chemokine analyses.
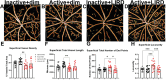
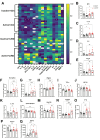
Results: Retinal arteriole and venule vasodilation was significantly increased in active + LIRD mice compared with inactive + LIRD mice. Superficial and intermediate/deep vascular plexi from inactive + LIRD mice had significantly decreased vessel density and total vessel length, with increased numbers of end points and lacunarity compared with active groups. Inactive + LIRD endothelial cells had significantly increased expression of VCAM1 and Nfkb1 compared with controls. Serum analyses revealed active + LIRD mice have a distinct immune response profile, with increased expression of keratinocyte-derived chemokine (KC), and VEGF-A compared to all other groups.
Conclusions: Treadmill exercise maintained retinal vascular morphology and function, altered endothelial gene expression, and is associated with a specific circulating immune response profile in the LIRD mouse model. These data support exercise as an effective intervention for maintaining retinal vascular integrity in a model of RD.
Conflict of interest statement
Disclosure:
Figures




Update of
-
Effects of treadmill exercise on retinal vascular morphology, function, and circulating immune factors in a mouse model of retinal degeneration.bioRxiv [Preprint]. 2025 Feb 12:2025.02.11.637694. doi: 10.1101/2025.02.11.637694. bioRxiv. 2025. Update in: Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):65. doi: 10.1167/iovs.66.11.65. PMID: 39990328 Free PMC article. Updated. Preprint.
References
-
- Davson H. The Bowman Lecture, 1979. The little brain. Trans Ophthalmol Soc U K (1962). 1979; 99(1): 21–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

