Wearable Ultrasound Devices for Therapeutic Applications
- PMID: 40856966
- PMCID: PMC12381330
- DOI: 10.1007/s40820-025-01890-2
Wearable Ultrasound Devices for Therapeutic Applications
Abstract
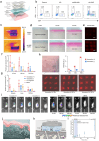
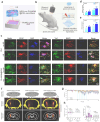
Wearable ultrasound devices represent a transformative advancement in therapeutic applications, offering noninvasive, continuous, and targeted treatment for deep tissues. These systems leverage flexible materials (e.g., piezoelectric composites, biodegradable polymers) and conformable designs to enable stable integration with dynamic anatomical surfaces. Key innovations include ultrasound-enhanced drug delivery through cavitation-mediated transdermal penetration, accelerated tissue regeneration via mechanical and electrical stimulation, and precise neuromodulation using focused acoustic waves. Recent developments demonstrate wireless operation, real-time monitoring, and closed-loop therapy, facilitated by energy-efficient transducers and AI-driven adaptive control. Despite progress, challenges persist in material durability, clinical validation, and scalable manufacturing. Future directions highlight the integration of nanomaterials, 3D-printed architectures, and multimodal sensing for personalized medicine. This technology holds significant potential to redefine chronic disease management, postoperative recovery, and neurorehabilitation, bridging the gap between clinical and home-based care.
Keywords: Closed-loop therapy; Drug delivery; Neurorehabilitation; Tissue regeneration; Wearable ultrasound devices.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- M. Lin, H. Hu, S. Zhou, S. Xu, Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7(11), 850–869 (2022). 10.1038/s41578-022-00427-y - DOI
-
- J. Chen, Y. Zhu, X. Chang, D. Pan, G. Song et al., Recent progress in essential functions of soft electronic skin. Adv. Funct. Mater. 31(42), 2104686 (2021). 10.1002/adfm.202104686 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
