Randomized investigation of heart failure therapy in patients with advanced cancer at risk of cardiac wasting: Rationale and design of the EMPATICC trial
- PMID: 40857084
- PMCID: PMC12575421
- DOI: 10.1002/ejhf.3799
Randomized investigation of heart failure therapy in patients with advanced cancer at risk of cardiac wasting: Rationale and design of the EMPATICC trial
Abstract
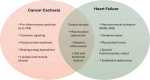
Aims: End-stage cancer may resemble a heart failure (HF)-like phenotype marked by cardiac wasting, dysfunction, and symptoms such as dyspnoea, congestion, and impaired physical function. The EMPATICC (EMPower the heArt of patients with TermInal Cancer using Cardiac medicines) trial evaluates the safety and efficacy of optimized HF therapy in patients with advanced cancer to improve self-care ability.
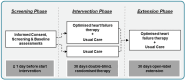
Methods: EMPATICC is a multicentre, investigator-initiated, randomized, double-blind, controlled, proof-of-concept trial employing a joint cardio-oncology care approach. Patients were randomized 1:1 to optimized HF therapy (sacubitril/valsartan, empagliflozin, ivabradine, ferric carboxymaltose) plus usual care, or usual care alone, for 30 days, followed by a 30-day open-label extension. Eligible patients had stage IV solid tumours (per Union for International Cancer Control), were receiving palliative care, had a 1-6 month life expectancy, and were on optimized analgesia. At baseline, first patients had to meet ≥2 criteria of the following indicating cardiovascular risk: heart rate ≥70 bpm, N-terminal pro-B-type natriuretic peptide ≥600 pg/ml, elevated high-sensitivity troponin, left ventricular ejection fraction <55%, left ventricular mass loss >15%, transferrin saturation <20%, or moderate/high likelihood of HF with preserved ejection fraction (based on the HFA-PEFF score); and they had to meet at least one criterion of the following indicating functional limitation: ≥6 s to walk 4 m, inability to wash ≥3 days of the last 7 days, or symptoms of dyspnoea at rest. Enrolment ended 30 January 2025; 93 patients completed randomization. The primary endpoint is a hierarchical composite (analysed by win ratio): (1) days alive and able to wash, (2) 4 m walking ability, and (3) patient global assessment of well-being.
Conclusions: EMPATICC evaluates whether HF therapy can improve function and well-being in advanced cancer, potentially reshaping care in this population.
Keywords: Cardiac wasting; Clinical trial; End‐stage cancer; Heart failure therapy.
© 2025 The Author(s). European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
References
-
- Surveillance, Epidemiology, and End Results Program ‐ National Cancer Institute . Cancer Stat Facts: Cancer of Any Site. 2024. Available from: https://seer.cancer.gov/statfacts/html/all.html.
-
- de Boer RA, Hulot JS, Tocchetti CG, Aboumsallem JP, Ameri P, Anker SD, et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:2272–2289. 10.1002/ejhf.2029 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

