The Novel Immunocompetent Eµ-SOX11CCND1 Mouse Model Phenotypically and Molecularly Resembles Human Mantle Cell Lymphoma
- PMID: 40857109
- PMCID: PMC12442894
- DOI: 10.1158/1078-0432.CCR-25-0534
The Novel Immunocompetent Eµ-SOX11CCND1 Mouse Model Phenotypically and Molecularly Resembles Human Mantle Cell Lymphoma
Abstract
Purpose: Mantle cell lymphoma (MCL) remains incurable despite therapeutic advances, highlighting the need for improved preclinical models. Existing transgenic MCL mouse models have significant limitations, restricting their translational value.
Experimental design: We generated an immunocompetent MCL model by overexpressing the key oncogenic drivers SRY-box transcription factor 11 (SOX11) and Cyclin D1 (CCND1) under the Eµ enhancer in C57BL/6 mice, aiming to replicate human MCL's biological and pathologic features.
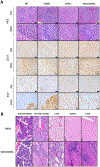
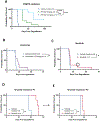
Results: Eµ-SOX11CCND1 mice developed lymphoma marked by clonal B1a cell expansion in lymphatic and extranodal tissues. Morphologic, immunophenotypic, and transcriptional profiling revealed strong similarity to human MCL, with pathway analysis confirming significant molecular overlap. Importantly, lymphoma cells could be adoptively transferred into wild-type recipients, enabling therapeutic testing within an intact immune system.
Conclusions: The Eµ-SOX11CCND1 mouse represents a robust and biologically relevant model that faithfully recapitulates human MCL. Its immunocompetent nature and adoptive transfer capability make it a valuable model for studying disease mechanisms and evaluating novel therapeutic approaches for patients with MCL.
©2025 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Conflicts of Interest
Employment: K.V. and P.S. are employees of Prelude Therapeutics
Stock: K.V. And P.S. have stock in Prelude Therapeutics
Research Support: R.A.B received research support from Prelude Therapeutics
S.P. has research support from Amgen, Celgene/Bristol Myers Squibb Corporation, Regeneron, Genentech, imCORE, Caribou and is a consultant for Grail (advisory board)
Figures




References
-
- Schieber M, Gordon LI, Karmali R. Current overview and treatment of mantle cell lymphoma. F1000Research. 2018;7.
-
- Saba NS, Liu D, Herman SE, Underbayev C, Tian X, Behrend D, et al. Pathogenic role of B-cell receptor signaling and canonical NF-κB activation in mantle cell lymphoma. Blood, The Journal of the American Society of Hematology. 2016;128(1):82–92.
-
- Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood, The Journal of the American Society of Hematology. 2016;127(12):1559–1563.
-
- Dreyling M, Aurer I, Cortelazzo S, Hermine O, Hess G, Jerkeman M, et al. Treatment for patients with relapsed/refractory mantle cell lymphoma: European-based recommendations. Leukemia & lymphoma. 2018;59(8):1814–1828. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

