Analysis of Long-term Follow-up of a Randomized Clinical Trial With Departures From Assigned Treatments: Estimation of Metformin Effects on Diabetes and Its Complications in the Diabetes Prevention Program Outcomes Study
- PMID: 40857175
- PMCID: PMC12451852
- DOI: 10.2337/dci25-0032
Analysis of Long-term Follow-up of a Randomized Clinical Trial With Departures From Assigned Treatments: Estimation of Metformin Effects on Diabetes and Its Complications in the Diabetes Prevention Program Outcomes Study
Abstract
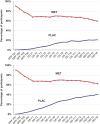
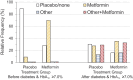
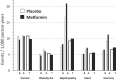
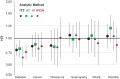
The Diabetes Prevention Program (DPP) was a 3-year randomized clinical trial (RCT) with evaluation of lifestyle and metformin interventions compared with placebo for diabetes prevention in high-risk adults. Both interventions significantly reduced diabetes incidence, prompting the long-term Diabetes Prevention Program Outcomes Study (DPPOS) to assess the progression of diabetes and its complications over 22 years. During follow-up, departures from the original metformin or placebo assignment occurred primarily because of development of diabetes that, by protocol, was managed by clinicians outside the study, after participants developed diabetes with HbA1c ≥7.0%. Diabetes development led to changes in metformin treatment and addition of other glucose-lowering therapies. Using statistical methods designed to estimate intervention effects despite these deviations, we consistently found that metformin reduced diabetes incidence. However, using these methods to evaluate whether use of metformin for prediabetes confers continued benefits after diabetes diagnosis did not substantially change the conclusions from those of the simpler intention-to-treat analysis that did not account for treatment changes. All of the analytic methods used resulted in similar metformin effect estimates with 95% CIs for hazard ratios including 1.0 (no effect) for all outcomes except for diabetes incidence. Elucidating metformin's long-term role in mitigating diabetes-related complications beyond its effects on diabetes prevention is challenging.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Sommer A, Zeger SL.. On estimating efficacy from clinical trials. Stat Med 1991;10:45–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK048375/DK/NIDDK NIH HHS/United States
- U01 DK048404, U01 DK048387, U01 DK048407/DK/NIDDK NIH HHS/United States
- U19 AG078558/AG/NIA NIH HHS/United States
- U01 DK048339/DK/NIDDK NIH HHS/United States
- U01 DK048380, U01 DK048397, U01 DK048412/DK/NIDDK NIH HHS/United States
- U01 DK048514, U01 DK048437, U01 DK048413/DK/NIDDK NIH HHS/United States
- U01 DK048349, U01 DK048381, U01 DK048468/DK/NIDDK NIH HHS/United States
- U01 DK048468/DK/NIDDK NIH HHS/United States
- U01 DK048387/DK/NIDDK NIH HHS/United States
- U01 DK048404/DK/NIDDK NIH HHS/United States
- U01 DK048377/DK/NIDDK NIH HHS/United States
- U01 DK048407/DK/NIDDK NIH HHS/United States
- U01 DK048437/DK/NIDDK NIH HHS/United States
- U01 DK048406/DK/NIDDK NIH HHS/United States
- 5 U19 AG078558/AG/NIA NIH HHS/United States
- U01 DK048412/DK/NIDDK NIH HHS/United States
- U01 DK048434/DK/NIDDK NIH HHS/United States
- U01 DK048443, and U01 DK048400/DK/NIDDK NIH HHS/United States
- U01 DK048411, U01 DK048406/DK/NIDDK NIH HHS/United States
- U01 DK048489, U01 DK048339, U01 DK048377/DK/NIDDK NIH HHS/United States
- U01 DK048413/DK/NIDDK NIH HHS/United States
- U01 DK048397/DK/NIDDK NIH HHS/United States
- U01 DK048381/DK/NIDDK NIH HHS/United States
- U01 DK048514/DK/NIDDK NIH HHS/United States
- U01 DK048485/DK/NIDDK NIH HHS/United States
- U01 DK048411/DK/NIDDK NIH HHS/United States
- U01 DK048443/DK/NIDDK NIH HHS/United States
- U01 DK048380/DK/NIDDK NIH HHS/United States
- U01 DK048434, U01 DK048485, U01 DK048375/DK/NIDDK NIH HHS/United States
- U01 DK048400/DK/NIDDK NIH HHS/United States
- U01 DK048489/DK/NIDDK NIH HHS/United States
- U01 DK048349/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

