Regulation of early gonocyte differentiation in zebrafish
- PMID: 40857739
- PMCID: PMC12493171
- DOI: 10.1042/BST20253046
Regulation of early gonocyte differentiation in zebrafish
Abstract
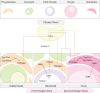
Zebrafish have been and continue to be an important model organism for studies of fundamental biology and biomedicine, including reproductive development and the cell intrinsic and extrinsic mechanisms regulating early gonocyte differentiation. Wild zebrafish strains determine sex using a ZW genetic system wherein the maternally inherited sex chromosome determines the embryo's sex. Like other species, including humans, regulation of conserved autosomal genes is crucial for gonocyte and sexual differentiation. How these conserved factors are regulated by the diverse mechanisms found throughout the animal kingdom is an active area of investigation. Domesticated zebrafish strains lack the ZW sex determination system found in wild strains and undergo gonocyte and sexual differentiation through a process exclusively governed by autosomal genes and nongenetic influences like environmental factors. Through mutational analysis, molecular genetics, and RNA sequencing, our understanding of the complexity of oocyte and spermatocyte differentiation has become clearer. In this review, we explore the most recent studies of the conserved and divergent mechanisms of gonocyte differentiation between wild and domesticated zebrafish as well as possible adaptations related to their domestication. Further, the contributions of individual genes and their molecular genetic hierarchy in regulating gonocyte differentiation are discussed and related to other species where relevant. We also address the recent characterization of a novel oocyte-progenitor and its potential implications in gonad differentiation. Finally, the role of gonocyte-extrinsic mechanisms, specifically communication between differentiating gonocytes and surrounding somatic gonad cells and the influence of resident and infiltrating immune cells, is discussed.
Keywords: bipotential gonad; gonadogenesis; indeterminant gonad; ovary; sexual differentiation; testis.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
LinkOut - more resources
Full Text Sources

