An age-related decrease in leptin contributes to CD8+ T cell aging in the tumor microenvironment
- PMID: 40858107
- PMCID: PMC12490250
- DOI: 10.1016/j.xcrm.2025.102310
An age-related decrease in leptin contributes to CD8+ T cell aging in the tumor microenvironment
Abstract
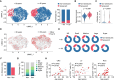
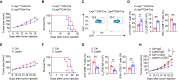
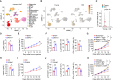
T cell dysfunction with age underlies an increased incidence of cancer in elderly individuals; however, how T cell aging is triggered in the tumor microenvironment is unclear. Here, we show that an age-associated reduction in adipocyte-derived leptin contributes to the accumulation of tumor-infiltrating senescent CD8+ T cells. Single-cell profiling of human and mouse cancer tissues reveals that the frequency of intratumoral senescent CD8+ T cells increases with age, leading to a weak antitumor effect. Moreover, decreased levels of adipocyte-derived leptin are an indispensable factor for CD8+ T cell aging. Leptin signaling prevents p38-dependent CD8+ T cell senescence. Furthermore, plasma leptin levels are negatively related to intratumoral CD8+ T cell senescence in cancer patients. Our findings identify an unappreciated interplay between metabolic perturbation and T cell aging and suggest that modulating adipocyte-derived leptin levels may be a promising therapeutic strategy for older cancer patients.
Keywords: CD8(+) T cells; T cell senescence; aging; antitumor immunity; leptin.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

