Different ranibizumab dosages for retinopathy of prematurity: 5-year follow-up data of the randomised, controlled CARE-ROP Study
- PMID: 40858340
- PMCID: PMC12382488
- DOI: 10.1136/bmjophth-2025-002303
Different ranibizumab dosages for retinopathy of prematurity: 5-year follow-up data of the randomised, controlled CARE-ROP Study
Abstract
Background: Data on long-term outcomes of antivascular endothelial growth factor therapy in retinopathy of prematurity (ROP) are still rare. We present 5-year post-treatment ophthalmological and paediatric outcomes of the prospective, multicentre, randomised, double-blinded, controlled pilot study CARE-ROP (Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity).
Methods: 14 patients (28 eyes) completed the ophthalmologic and 5 patients completed the paediatric 5-year follow-up assessment. The ophthalmological assessment included best-corrected visual acuity (BCVA), orthoptic status, slit lamp examination, intraocular pressure and funduscopy. The paediatric examination followed the German Neonatal Network protocol and covered cognitive, motor and sensory development.
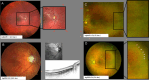
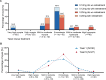
Results: 17 of 28 eyes exhibited at least one ocular abnormality, such as optic disc pallor or atrophy of the optic nerve head, retinal pigment epithelium (RPE) pigment changes, persistent tortuosity or macular hypoplasia. Despite this, 19 of 26 eyes demonstrated a logarithm of the Minimum Angle of Resolution (logMAR) BCVA of 0.3 or better. Mean refractive error was -0.9 D (±3.4 D) with only two eyes of one infant having high myopia of < -5 D. The neurodevelopmental results were within the expected range for a population of preterm infants with treatment-warranting ROP but need to be interpreted with caution due to the low number of five patients.
Conclusion: The 5-year ophthalmologic and paediatric outcomes of the CARE-ROP study confirm the previous results and add important additional information on long-term safety of 0.12 and 0.20 mg ranibizumab for the treatment of ROP. The ophthalmologic functional outcome regarding BCVA and refraction is promising. These exploratory long-term data, however, need to be interpreted with caution due to the low patient number both in the ophthalmologic and paediatric assessment.
Keywords: Child health (paediatrics); Retina.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AS: Speaker fees/honoraria from Allergan, Astellas, Bayer, Novartis, Roche; research funding from Novartis, Bayer and Roche. M-CB: Speaker fees from Alcon, consultancy reimbursement from AbbVie. WL: Speaker fees/honoraria Santhera, Infectopharm, Boehringer Ingelheim. FEM, TB and NE: None declared. RG: Honoraria from Bayer and Roche. TUK: Lecture fees/travel reimbursements/consultancy honoraria from AbbVie, Alimera, Bayer, Heidelberg Engineering, Novartis, Roche, Stada; financial research support from Bayer, Novartis. WG: Speaker fees from Chiesi Farmaceutici. JMP: Speaker fees from Novartis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
