Multiplex bead assays enable integrated serological surveillance and reveal cross-pathogen vulnerabilities in Zambezia Province, Mozambique
- PMID: 40858567
- PMCID: PMC12381283
- DOI: 10.1038/s41467-025-62305-9
Multiplex bead assays enable integrated serological surveillance and reveal cross-pathogen vulnerabilities in Zambezia Province, Mozambique
Erratum in
-
Author Correction: Multiplex bead assays enable integrated serological surveillance and reveal cross-pathogen vulnerabilities in Zambezia Province, Mozambique.Nat Commun. 2025 Oct 7;16(1):8901. doi: 10.1038/s41467-025-64869-y. Nat Commun. 2025. PMID: 41057351 Free PMC article. No abstract available.
Abstract
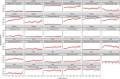
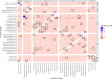
Multiplex serological assays simultaneously measure antibodies to multiple antigens, furnishing insights into exposure and susceptibility to several pathogens and cross-pathogen vulnerabilities. Our serosurvey tests dried blood spots from 1292 individuals for IgG antibodies to 35 antigens from 18 pathogens using a multiplex bead assay for vaccine preventable diseases, malaria, SARS-CoV-2, neglected tropical diseases, and enteric pathogens in Mozambique. We produce pathogen-specific seroprevalence estimates and age-seroprevalence curves and identify spatial differences in seroprevalence. Rural clusters have higher odds of seropositivity to most NTDs neglected tropical diseases, Plasmodium falciparum malaria, and enteric pathogens, but lower odds of seropositivity to SARS-CoV-2 and vaccine preventable diseases compared to urban clusters. This co-occurrence identifies clusters with high vulnerability to multiple pathogens. We identify a candidate group of antigens that are correlated with high overall vulnerability. Our results demonstrate a role for multiplex serology in integrated disease surveillance to guide control strategies for individual and co-endemic pathogens.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: The research included local researchers throughout the research process. The research was determined in collaboration with local partners and has been presented to the local authorities. Roles and responsibilities were agreed among collaborators ahead of the research. This research was not severely restricted or prohibited in the setting of the researchers. Any risk of stigmatization, incrimination, discrimination, or personal risk to participants was minimized through local ethical considerations, including compliance with anonymity, privacy, and referrals to health facilities as dictated by local Ministry of Health policies. The study protocol was approved by the National Bioethics Committee for Health of Mozambique and the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Figures



References
-
- Cutts, F. T. & Hanson, M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health21, 1086–1098 (2016). - PubMed
-
- Carcelen, A. K., Hegde, A., Takahashi, S., Moss, S. & W. J. Serosurveillance Summit Meeting Report (Baltimore, MD, USA: Johns Hopkins Bloomberg School of Public Health International Vaccine Access Center, 2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

