Nascent liver proteome reveals enzymes and transcription regulators under physiological and alcohol exposure conditions
- PMID: 40858584
- PMCID: PMC12381119
- DOI: 10.1038/s41467-025-63212-9
Nascent liver proteome reveals enzymes and transcription regulators under physiological and alcohol exposure conditions
Abstract
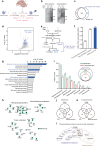
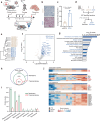
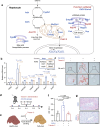
The liver proteome undergoes dynamic changes while performing hundreds of essential biological functions. Dysregulation of the liver proteome under alcoholic conditions leads to alcohol-associated liver disease (ALD), a major health challenge worldwide. There is an urgent need for quantitative and liver-specific proteome information in living animals to understand the pathophysiological dynamics of this largest solid organ. Here, we develop a comprehensive approach that specifically identifies the nascent proteome and preferentially enriches membrane proteins in living mouse hepatocytes and is broadly applicable to studies of the liver under various physiological and pathological conditions. In the ethanol-induced liver injury mouse model, the nascent proteome successfully identifies and validates a number of transcription regulators, enzymes, and protective chaperones involved in the molecular regulation of hepatic steatosis, in addition to almost all known regulatory proteins and pathways related to alcohol metabolism. We discover that Phb1/2 is an important transcription coregulator in the process of ethanol metabolism, and one identified fatty acid metabolism enzyme Acsl1/5, whose inhibition protects cells and mice from lipid accumulation, a key symptom of hepatic steatosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

