Helicobacter hepaticus promotes hepatic steatosis through CdtB-induced mitochondrial stress and lipid metabolism reprogramming
- PMID: 40858606
- PMCID: PMC12381262
- DOI: 10.1038/s41467-025-63351-z
Helicobacter hepaticus promotes hepatic steatosis through CdtB-induced mitochondrial stress and lipid metabolism reprogramming
Abstract
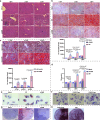
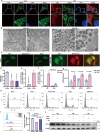
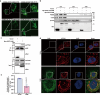
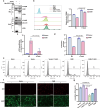
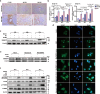
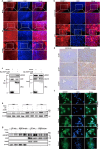
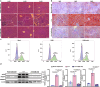
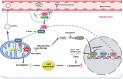
Host-pathogen interaction influences many non-infectious diseases, including metabolic diseases. Helicobacter hepaticus (H. hepaticus) has been found in some metabolic dysfunction-associated steatotic liver disease (MASLD) patients, however, the causal link and underlying mechanisms remain unclear. Here we report that H. hepaticus infection or overexpression of CdtB of H. hepaticus induces lipid deposition in hepatocytes, both in vivo and in vitro. Furthermore, we identify that CdtB translocates to mitochondria with the help of Hsp90, interacts with ATP5A1, reduces mitochondrial respiratory complex V activity, damages mitochondria, and disrupts lipid metabolism. Mechanistically, CdtB-induced lipogenesis depends on the CdtB-mitochondrial ROS-mTORC1-SREBP1 axis and CdtB-mediated NONO expression to enhance nuclear localization of SREBP1 that promote the de novo fatty acid synthesis in the hepatocytes. Neutralization of CdtB significantly alleviates hepatic lipidosis in mice upon H. hepaticus infection. Furthermore, the nucleic acid of H. hepaticus has been detected in the liver tissues of some patients with MASLD, which suggests a certain correlation between liver infection with H. hepaticus and the occurrence and progression of MASLD. Our findings highlight the critical role of CdtB in the pathogenesis of H. hepaticus infection-induced hepatic lipidosis and its potential as a therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Huttasch, M., Roden, M. & Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease?. Metabolism157, 155937 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

