Differential role of C-terminal truncations on alpha-synuclein pathology and Lewy body formation
- PMID: 40858624
- PMCID: PMC12381302
- DOI: 10.1038/s41531-025-01084-y
Differential role of C-terminal truncations on alpha-synuclein pathology and Lewy body formation
Abstract
Alpha-synuclein (aSyn) post-translational modifications (PTM), especially phosphorylation at serine 129 and C-terminal truncations, are highly enriched in Lewy bodies (LB), Lewy neurites, and other pathological aggregates in Parkinson's disease and synucleinopathies. However, the precise role of these PTM in pathology formation, neurodegeneration, and pathology spreading remains unclear. Here, we systematically investigated the role of post-fibrillization C-terminal aSyn truncations in regulating uptake, processing, seeding, and LB-like inclusion formation using a neuronal seeding model that recapitulates LB formation and neurodegeneration. We show that C-terminal cleavage of aSyn fibrils occurs rapidly post exogenous fibril internalization and during intracellular LB-like inclusion formation. Blocking cleavage of internalized fibrils does not affect seeding, but inhibiting enzymes such as calpains 1 and 2 alters LB-like inclusion formation. We show that C-terminal truncations, along with other PTMs, regulate fibril interactome remodeling, shortening, lateral association, and packing. These findings reveal distinct roles of C-terminal truncations at different aggregation stages on the pathway to LB formation, highlighting the need for consideration of stage‑specific strategies to target aSyn proteolytic cleavages.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.M.C., A.M., P.D., and M.C. are employees of UCB Pharma. H.A.L. is the co-founder and chief scientific officer of ND BioSciences, Epalinges, Switzerland, a company that develops diagnostics and treatments for neurodegenerative diseases (NDs) based on platforms that reproduce the complexity and diversity of proteins implicated in NDs and their pathologies. H.A.L. has received funding from industry to support research on neurodegenerative diseases, including Merck Serono, UCB, and AbbVie. These companies had no specific role in the conceptualization, preparation, and decision to publish this manuscript. H.A.L. is the associate editor of npj Parkinson’s Disease. H.A.L. was not involved in the journal’s review of, or decisions related to, this manuscript. All other authors declare no competing financial interests in association with this manuscript.
Figures

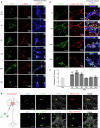

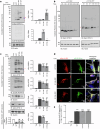



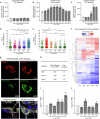
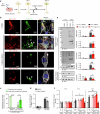

References
-
- Anderson, J. P. et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem.281, 29739–29752 (2006). - PubMed
-
- Fujiwara, H. et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol.4, 160–164 (2002). - PubMed
-
- Crowther, R. A., Jakes, R., Spillantini, M. G. & Goedert, M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett.436, 309–312 (1998). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

