Oxic microbial ferrihydrite reduction rates of Shewanella oneidensis and the potential for Fe mobilization in oxic sediments
- PMID: 40858809
- PMCID: PMC12381104
- DOI: 10.1038/s41598-025-16963-w
Oxic microbial ferrihydrite reduction rates of Shewanella oneidensis and the potential for Fe mobilization in oxic sediments
Abstract
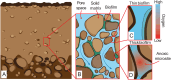
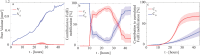
Microbially mediated reduction of ferrihydrite (Fe(III) oxyhydroxide) plays a crucial role in Fe cycling, and hence nutrient and contaminant cycling, in subsurface environments. This process is typically considered a strictly anaerobic process confined to anoxic microsites within oxic subsurface environments. However, recent findings suggest that microbes can also mediate ferrihydrite reduction under oxic conditions. Here, we quantified cell-specific rates of ferrihydrite reduction by the model organism Shewanella oneidensis MR-1 under oxic and anoxic conditions. We used these rates to assess the relative contribution of oxic and anoxic pore spaces to Fe(II) mobilization in a previously published laboratory analog of oxic aquifer sediments. Oxic reduction proceeded persistently, albeit at a per cell rate 100 times more slowly than anoxic reduction, yet still generated appreciable Fe(II). Modeling suggests that when anoxic microsites are absent or occupy a minor fraction of the pore space, oxic Fe(III) reduction can account for a significant share of total Fe(II) release. Such conditions are common in shallow aquifers, well-drained soils, and capillary fringes. We conclude that oxic Fe(III) reduction is a persistent background process that has been underestimated in current biogeochemical frameworks.
Supplementary Information: The online version contains supplementary material available at 10.1038/s41598-025-16963-w.
Keywords: Shewanella oneidensis; Anoxic microsites; Ferrihydrite; Microbial iron reduction; Oxic sediments; Oxygen.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Kappler, A. et al. An evolving view on biogeochemical cycling of iron. Nat. Rev. Microbiol.19 (6), 360–374 (2021). - PubMed
-
- Pallud, C., Masue-Slowey, Y. & Fendorf, S. Aggregate-scale Spatial heterogeneity in reductive transformation of ferrihydrite resulting from coupled biogeochemical and physical processes. Geochim. Cosmochim. Acta. 74 (10), 2811–2825 (2010).
-
- Huang, J. et al. Fe (II) redox chemistry in the environment. Chem. Rev.121 (13), 8161–8233 (2021). - PubMed
-
- Dixit, S. & Hering, J. G. Comparison of Arsenic (V) and Arsenic (III) Sorption Onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environmental science & technology, 37(18), 4182-4189 (2003). - PubMed
-
- Zhao, Y. et al. The mobility and fate of cr during aging of ferrihydrite and ferrihydrite organominerals. Geochim. Cosmochim. Acta. 347, 58–71 (2023).
Grants and funding
LinkOut - more resources
Full Text Sources

