Mevalonate metabolites boost aged oocyte quality through prenylation of small GTPases
- PMID: 40858817
- PMCID: PMC12532580
- DOI: 10.1038/s43587-025-00946-7
Mevalonate metabolites boost aged oocyte quality through prenylation of small GTPases
Abstract
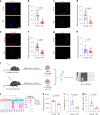
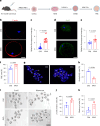
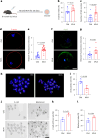
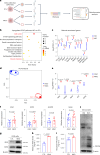
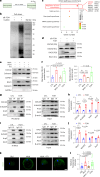
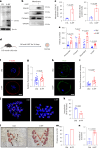
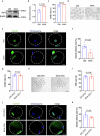
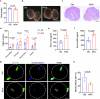
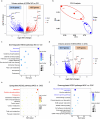
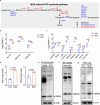
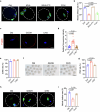
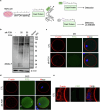
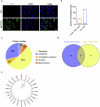
Declining oocyte quality is the major contributor to female subfertility in aged mammals. Currently, there are no effective interventions to ameliorate aged oocyte quality. Here we found that oocytes at metaphase I from the cumulus-oocyte complexes of aged mice showed reduced cortical F-actin and lower levels of mevalonate (MVA) pathway metabolites, including MVA, farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate. We further showed that MVA supplementation improved FPP levels, cortical F-actin and the quality of aged oocytes. Mechanistically, we found that MVA supplementation induced granulosa cells to synthesize FPP, which was subsequently transferred to aged oocytes. Transported FPP increased the prenylation of small GTPases, including CDC42 and RAC1, and promoted membrane localization of CDC42-N-WASP-Arp2/3 and RAC1-WAVE2-Arp2/3 complexes, promoting cortical F-actin reassembly and reducing aneuploidy of aged oocytes. We also identified a natural chemical compound, 8-isopentenyl flavone, with an isopentenyl side chain from Epimedium brevicornu Maxim, which could increase CDC42 and RAC1 prenylation, improving the cortical F-actin and the competence of aged oocytes, and ameliorating reproductive outcomes in aged female mice. Collectively, increasing the prenylation of small GTPases via MVA metabolites or 8-isopentenyl flavone provides a therapeutic approach for boosting female fertility during reproductive aging.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

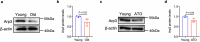


References
-
- Broekmans, F. J., Soules, M. R. & Fauser, B. C. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev.30, 465–493 (2009). - PubMed
-
- Laisk, T. et al. Demographic and evolutionary trends in ovarian function and aging. Hum. Reprod. Update25, 34–50 (2019). - PubMed
-
- Perheentupa, A. & Huhtaniemi, I. Aging of the human ovary and testis. Mol. Cell. Endocrinol.299, 2–13 (2009). - PubMed
-
- Nikalayevich, E. et al. Aberrant cortex contractions impact mammalian oocyte quality. Dev. Cell59, 841–852.e7 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

