Inhibition of proprotein convertase SKI-1 prevents blood vessel alteration after stroke
- PMID: 40858841
- PMCID: PMC12436164
- DOI: 10.1038/s44161-025-00691-5
Inhibition of proprotein convertase SKI-1 prevents blood vessel alteration after stroke
Abstract
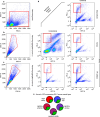
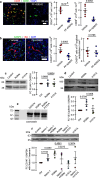
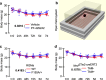
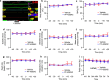
Neutralizing factors involved in blood vessel dysfunction offer a promising strategy for stroke recovery. Many extracellular proteins need enzymatic activation to function, and blocking this activation is an untapped approach to restoring vessel integrity. Here we demonstrate that inhibition of the extracellular protease SKI-1 with PF-429242 restores blood vessel integrity and promotes functional recovery in both large and small animal models for stroke. Single-cell mRNA sequencing identified molecular signatures suggesting that PF-429242 restores the expression of genes involved in vessel integrity in endothelial cells. Moreover, we identify a mechanism whereby RGMa cleavage by SKI-1 is required for RGMa to interact with Neogenin and alter vessel integrity. Either preventing RGMa cleavage or deleting Neogenin on endothelial cells reduced blood vessel dysfunction, increased tissue preservation and restored brain function after stroke. This work identifies a much-needed therapeutic strategy that restores blood vessel integrity and functionality, showing efficacy in large and small animals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures













References
-
- Martin, M. et al. Engineered Wnt ligands enable blood–brain barrier repair in neurological disorders. Science375, eabm4459 (2022). - PubMed
-
- Li, M. et al. Adenoviral vector-induced silencing of RGMa attenuates blood-brain barrier dysfunction in a rat model of MCAO/reperfusion. Brain Res. Bull.142, 54–62 (2018). - PubMed
-
- Marcinkiewicz, M., Seidah, N. G. & Chrétien, M. Implications of the subtilisin/kexin-like precursor convertases in the development and function of nervous tissues. Acta Neurobiol. Exp. (Wars.)56, 287–298 (1996). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
