Acid-less direct gram scale exfoliation of graphite to partially oxidized graphene
- PMID: 40858911
- PMCID: PMC12381218
- DOI: 10.1038/s41598-025-99502-x
Acid-less direct gram scale exfoliation of graphite to partially oxidized graphene
Abstract
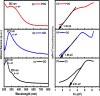
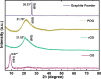
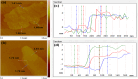
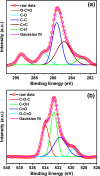
All methods to synthesize graphene oxide on a large scale utilize a strongly acidic medium. Graphene oxide (GO) to reduced graphene oxide (rGO) conversion, i.e., obtaining widespread sp2-sp2 hybridized carbon allotropes is a laborious and tedious process. Herein, we report an innovative one-step method, through which we fabricate 'partially oxidized graphene' on a gram scale directly from graphite powder, without using any acids. The emergence of the (002) XRD peak, band gap (Eg ~ 1.42 ± 0.01 eV), and carbon-to-oxygen (C/O) ratio of 3.67 validate the direct POG phase formation without any reduction step. Raman (ID/IG ~ 0.80 ± 0.06) and AFM studies reveal the less defective and bi- to few-layer character of POG sheets of thickness ca. 1.59 ± 0.14 nm. The measured conductivity of the POG material (9.22 ± 0.04 S/cm) is found to be higher than the conductivity of the rGO using the standard method (0.30 ± 0.03 S/cm). The zeta potential study suggests a good stability of the POG aqueous dispersion. Furthermore, the proposed method is efficient (2-3 h), safe, and renders a higher yield (77%) than many conventional methods (40-70%) for GO preparation. This offers a promising source for conductive electrodes, carbon paste, and more.
Keywords: Acid-less; Aqueous medium; Exfoliation; Graphene; Partially oxidized graphene.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Cathodic Exfoliation of Various Graphite Materials in Potassium Chloride Electrolyte.Molecules. 2025 Jul 28;30(15):3151. doi: 10.3390/molecules30153151. Molecules. 2025. PMID: 40807326 Free PMC article.
-
Synthesis of a covalently linked bismuthene-graphene heterostructure loaded with mitomycin C for combined radio-thermo-chemotherapy of triple-negative breast cancer.J Mater Chem B. 2025 Jul 2;13(26):7769-7784. doi: 10.1039/d5tb00096c. J Mater Chem B. 2025. PMID: 40468814
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
-
- Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science306(5696), 666–669 (2004). - PubMed
-
- Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater.6(3), 183–191 (2007). - PubMed
-
- Hummers, W. S. Jr & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc.80(6), 1339–1339 (1958).
-
- Pei, S. & Cheng, H. M. The reduction of graphene oxide. Carbon50(9), 3210–3228 (2012).
-
- Bacon, M., Bradley, S. J. & Nann, T. Graphene quantum dots. Part. Part. Syst. Charact.31(4), 415–428 (2014).
LinkOut - more resources
Full Text Sources
Miscellaneous

