Revealing the protective potential of D-(-)-Quinic acid against thioacetamide-induced hepatic encephalopathy in rats
- PMID: 40858982
- PMCID: PMC12381148
- DOI: 10.1038/s41598-025-17356-9
Revealing the protective potential of D-(-)-Quinic acid against thioacetamide-induced hepatic encephalopathy in rats
Abstract
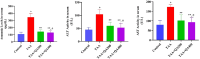
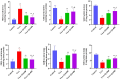
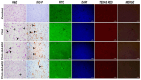
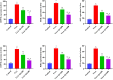
The objective of this study was to elucidate the impact of D-(-)-Quinic acid (D-(-)-QA), a natural phenolic acid, on the molecular mechanisms of inflammation and oxidative stress in brain and liver damage in thioacetamide (TAA)-induced hepatic encephalopathy (HE) in rats. To establish an experimental HE model, rats were injected with three doses (200 mg/kg, intraperitoneally) of TAA every other day, followed by the administration of D-(-)- QA (200 or 400 mg/kg, orally) for fourteen days. Serum ammonia, alanine transaminase (ALT), and aspartate transaminase (AST) levels were detected. Tumor necrosis factor (TNF-α), interleukin-1beta IL-1β, and malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) levels were analyzed to assess inflammation and oxidative damage in brain and liver tissues. Histopathological examinations, immunofluorescence (PI3K and AKT expressions), and immunohistochemical (HSP70 expression) examinations were also performed. TAA administration causedhyperammonemia and increased ALT and AST levels, along with histopathological changes indicating damage to hepatocytes and neurons. Additionally, it disrupted the inflammatory response and promoted oxidative damage by activating the PI3K/AKT-related HSP70 response in the brain and liver. Treatment with D-(-)-QA significantly prevented the increase in ammonia levels and improved the liver function. In addition, the increase in TNF-α, and IL-1β, proinflammatory cytokines in liver and brain tissues as well as the increase in MDA and decrease in SOD and GSH were significantly suppressed by D-(-)-QA. By suppressing HSP70 and PI3K/AKT signaling in brain and liver tissues, D-(-)-QA administration attenuated inflammation and oxidative damage. D-(-)-QA may be an alternative to current clinical interventions by inhibiting inflammation and oxidative damage signaling toward the pathogenesis of HE.
Keywords: D-(-)-Quinic acid; HSP70; Hepatic encephalopathy; Inflammation; Oxidative stress; PI3K/AKT signaling; Thioacetamide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study protocol was approved by Erzincan Binali Yıldırım University Experimental Animal Ethics Committee with the decision dated 31.10.2024 and numbered 47. All experiments were carried out in accordance with European Parliament Directive 2010/63/EU (Approval Number 2016-24-199) and ARRIVE guidelines.
Figures





References
-
- Tranah, T. H., Vijay, G. K., Ryan, J. M. & Shawcross, D. L. Systemic inflammation and ammonia in hepatic encephalopathy. Metab. Brain Dis.28, 1–5 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

