Predetermined sex revealed by a female transient gut in non-feeding larvae of Osedax (Siboglinidae, Annelida)
- PMID: 40859373
- PMCID: PMC12382133
- DOI: 10.1186/s13227-025-00251-9
Predetermined sex revealed by a female transient gut in non-feeding larvae of Osedax (Siboglinidae, Annelida)
Abstract
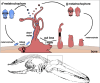
Background: Within the symbiont-hosting Siboglinidae (Annelida), Osedax stands out as the sole genus capable of degrading bones and displaying pronounced sexual dimorphism (except O. priapus). While macroscopic, gutless females feed on whale falls with their symbiont-housing "roots", males are microscopic and non-feeding. Yet, embryos and larvae look identical, and sex is suggested to be environmentally determined, i.e., larvae metamorphose into females on bare bone or into males when finding an adult female.
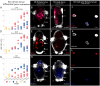
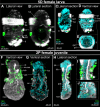
Results: However, we here describe a transient gut present in half of the late larvae and in juvenile females of O. japonicus. We confirm the gut-carrying larvae as being females from sex-specific in situ gene expression. Moreover, morphological evidence coupled with differential gene expression indicate that the 'non-feeding' transient gut may pattern the vascular system and/or act as a gas-exchange surface in juvenile females, before their branchial appendages develop.
Conclusions: The transient gut of O. japonicus females reveals a genetic sex determination. Proposedly homologous across siboglinids, this vestigial gut is suggested to function in organ patterning and/or for gas-exchange during development of the gutless adult.
Keywords: Lecithotrophic larvae; Rudimentary gut; Sex determination; Sexual dimorphism; Trochophore; Vascular system.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


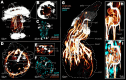
References
-
- Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB. Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science. 1981;213(4505):340–2. - PubMed
-
- Rouse GW, Goffredi SK, Vrijenhoek RC. Osedax : bone-eating marine worms with dwarf males. Science. 2004;305(5684):668–71. - PubMed
-
- Southward EC, Schulze A, Gardiner SL. Pogonophora (Annelida): form and function. Hydrobiologia. 2005;535(536):227–51.
-
- Goffredi SK, Orphan VJ, Rouse GW, Jahnke L, Embaye T, Turk K, Lee R, Vrijenhoek RC. Evolutionary innovation: a bone-eating marine symbiosis. Environ Microbiol. 2005;7:1369–78. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

