Simultaneous electrochemical detection of Cd2+ and Pb2+ using a green silver nanoparticles/polyaniline-modified carbon paste electrode
- PMID: 40860078
- PMCID: PMC12377198
- DOI: 10.1039/d5ra03135d
Simultaneous electrochemical detection of Cd2+ and Pb2+ using a green silver nanoparticles/polyaniline-modified carbon paste electrode
Abstract
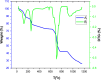
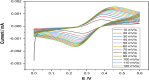
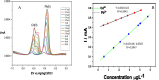
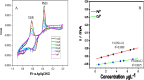
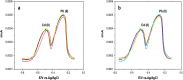
In this study, a novel electrochemical sensor based on a carbon paste electrode (CPE) modified with polyaniline (PANI) and green-synthesized silver nanoparticles (AgNPs) was developed for the simultaneous detection of cadmium (Cd2+) and lead (Pb2+) ions in aqueous solutions. The AgNPs were synthesized using a green route employing plant extract as a reducing and capping agent, ensuring environmental sustainability. The modified electrode (AgNPs-PANI-CPE) was characterized by UV-Vis spectroscopy, field-emission gun scanning electron microscopy (FEG-SEM), and simultaneous thermal analysis (TGA/DSC). The electrochemical behavior of Cd2+ and Pb2+ was investigated using square wave voltammetry (SWV) and CV. The sensor exhibited distinct and well-separated anodic peaks for Cd2+ and Pb2+, with excellent sensitivity, wide linear response ranges, and low detection limits (0.09 and 0.05 μg L-1, respectively). Interference studies demonstrated good selectivity towards the target ions, and successful application to real water samples confirmed its analytical performance. This work highlights the potential of eco-friendly nanocomposite-modified electrodes in environmental monitoring of toxic heavy metals.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

