Influence of hydrogen bonding on twisted intramolecular charge transfer in coumarin dyes: an integrated experimental and theoretical investigation
- PMID: 40860080
- PMCID: PMC12376945
- DOI: 10.1039/d5ra02669e
Influence of hydrogen bonding on twisted intramolecular charge transfer in coumarin dyes: an integrated experimental and theoretical investigation
Abstract
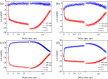
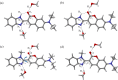
Twisted intramolecular charge transfer (TICT) is a critical mechanism influencing the emission efficiency and stability of fluorescent materials, thereby playing a pivotal role in the design of highly fluorescent and stable dyes. Although substantial research has concentrated on the role of intermolecular hydrogen bonding in excited-state dynamics, the impact of intramolecular hydrogen bonding has not been thoroughly investigated. To elucidate the solvent polarity dependence of C7 and C30, we employed the Kamlet-Taft and Catalán 4P models in conjunction with steady-state and transient absorption spectroscopy, complemented by time-dependent density functional theory (TDDFT) calculations. Our findings demonstrate that C30 exhibits a pronounced TICT process in both solvents. Conversely, C7, stabilized by intramolecular hydrogen bonds, retains a planar configuration of its benzimidazole and benzopyrone moieties, effectively preventing the TICT process. Moreover, in MeOH, the intermolecular hydrogen bonding in C30 significantly extends the lifetime of the TICT state compared to ACN. Theoretical analyses of electrostatic potential, molecular geometry, and frontier molecular orbitals further corroborate these observations. This work provides valuable insights into the design of fluorescent dye molecules and the selection of solvents, laying a foundation for advancing the photophysical and photochemical understanding of coumarin dyes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
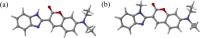




References
-
- Hanaoka K. Iwaki S. Yagi K. Myochin T. Ikeno T. Ohno H. Sasaki E. Komatsu T. Ueno T. Uchigashima M. Mikuni T. Tainaka K. Tahara S. Takeuchi S. Tahara T. Uchiyama M. Nagano T. Urano Y. J. Am. Chem. Soc. 2022;144:19778–19790. - PubMed
-
- Zhao G. J. Han K. L. Phys. Chem. Chem. Phys. 2010;12:8914–8918. - PubMed
-
- Zhang H. Zeitz D. C. Zhang J. Z. J. Phys. Chem. Lett. 2023;14:8095–8099. - PubMed
-
- Venkatraman R. K. Orr Ewing A. J. J. Am. Chem. Soc. 2019;141:15222–15229. - PubMed
-
- Wang C. Qiao Q. L. Chi W. J. Chen J. Liu W. J. Tan D. McKechnie S. Lyu D. Jiang X. F. Zhou W. Xu N. Zhang Q. S. Xu Z. C. Liu X. G. Angew. Chem., Int. Ed. 2020;59:10160–10172. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

