Engineered upconversion nanoparticles for breast cancer theranostics
- PMID: 40860139
- PMCID: PMC12374582
- DOI: 10.7150/thno.116153
Engineered upconversion nanoparticles for breast cancer theranostics
Abstract
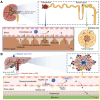
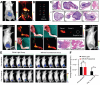
Breast cancer (BC) remains the most prevalent cancer among women and a leading cause of cancer-related mortality worldwide, posing a significant threat to public health. Rare earth (RE)-doped upconversion nanoparticles (UCNPs) have emerged as a promising nanoplatform for BC management, owing to their exceptional photophysical properties and design flexibility. Unlike conventional fluorescent probes, engineered UCNPs absorb near-infrared (NIR) light, enabling deep tissue penetration while mitigating tissue damage and spontaneous fluorescence interference. Furthermore, through core-shell structure engineering and functionalization, multiple diagnostic and therapeutic modules can be integrated within a single NP, enabling theranostic applications for BC. This review comprehensively summarizes recent advances in engineered UCNPs for BC theranostics. It begins by introducing the luminescence mechanisms, controllable synthesis methods, and surface modification strategies of UCNPs. Next, it explores the fundamental biological effects of UCNPs, including biodistribution, metabolic pathways, and biotoxicity. Subsequently, we systematically review applications of engineered UCNPs in BC molecular imaging, biomarker detection, phototherapy, smart drug/gene delivery, and immunotherapy. Finally, current challenges and clinical translation prospects of UCNPs are discussed.
Keywords: biomarker detection; breast cancer; delivery; immunotherapy; molecular imaging; phototherapy; theranostics; upconversion nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

















References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Kim J, Harper A, McCormack V, Sung H, Houssami N, Morgan E. et al. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat Med. 2025;31:1154–62. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Heck L, Herzen J. Recent advances in X-ray imaging of breast tissue: from two- to three-dimensional imaging. Phys Medica PM Int J Devoted Appl Phys Med Biol Off J Ital Assoc Biomed Phys AIFB. 2020;79:69–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

