PLK4 as a Key Regulator of Neuroblastoma Differentiation and a Promising Therapeutic Target
- PMID: 40860200
- PMCID: PMC12374821
- DOI: 10.7150/ijbs.111449
PLK4 as a Key Regulator of Neuroblastoma Differentiation and a Promising Therapeutic Target
Abstract
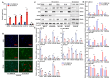
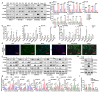
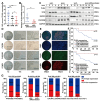
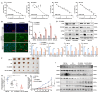
Background: Neuroblastoma (NB) differentiation status critically influences prognosis and treatment response. Although differentiation therapy has shown clinical benefit, its efficacy remains limited. The molecular mechanisms driving NB differentiation are not fully understood. PLK4 has been linked to NB tumorigenesis, but its role in regulating differentiation remains unclear. Methods: We investigated the role of PLK4 in neuroblastoma differentiation by modulating its expression both in vitro and in vivo. Through comprehensive analyses employing Western blotting, co-immunoprecipitation, immunofluorescence and murine neuroblastoma models, we identified downstream signaling pathways involved in PLK4-mediated regulation of neuronal genes. Pharmacological inhibition of PLK4 further confirmed its functional relevance in promoting neuroblastoma differentiation. Results: PLK4 functions as a key regulator of neuroblastoma differentiation. Its depletion enhances neuronal maturation and sensitizes cells to 13-cis RA. Mechanistically, we identify a novel PLK4-CXCR4 signaling axis that governs neuroblastoma differentiation through PI3K/Akt-mediated modulation of cyclin D1 expression. The selective PLK4 inhibitor CFI-400945 exhibits dual anti-tumor activity by promoting terminal differentiation and suppressing proliferation. Conclusions: Our study identifies PLK4 as a potential molecular switch governing NB differentiation and a promising therapeutic target to overcome resistance to 13-cis RA.
Keywords: CXCR4; PLK4; cyclin D1; differentiation therapy; neuroblastoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- van Groningen T, Koster J, Valentijn LJ. et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. 2017;49:1261–1266. - PubMed
-
- Qiu B, Matthay KK. Advancing therapy for neuroblastoma. Nat Rev Clin Oncol. 2022;19:515–533. - PubMed
-
- Matthay KK, Maris JM, Schleiermacher G. et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

