Fracture-Induced Immunological Cascades Trigger Rapid Systemic Bone Loss via Osteocyte-Regulated Osteoclastogenesis
- PMID: 40860346
- PMCID: PMC12377381
- DOI: 10.2147/ITT.S533552
Fracture-Induced Immunological Cascades Trigger Rapid Systemic Bone Loss via Osteocyte-Regulated Osteoclastogenesis
Abstract
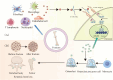
Background: Rapid bone loss after fracture elevates the risk of subsequent fractures, but the mechanisms remain unclear. IL-6, a key cytokine involved in fracture healing, is markedly upregulated during the immune response after fracture; however, its role in systemic skeletal deterioration remains poorly defined.
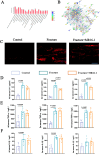
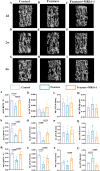
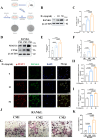
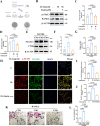
Methods: In this study, we employed label-free proteomics to identify candidate mediators in vertebral samples following fracture. Next, osteocyte siRNA knockdown and Stattic (STAT3 phosphorylation inhibitor) inhibition were used to investigate IL-6 related signaling pathways. Subsequently, indirect co-cultures of osteocyte with osteoclast or osteoblast were used to evaluate the effects of the IL-6 pathway on bone resorption and formation. Furthermore, fractured mice were treated with MR16-1 (monoclonal anti-mouse IL-6 receptor antibody) or Stattic. Then, trabecular and cortical bone in vertebrae and femur were evaluated at 4, 14, and 28 days post-fracture, including histological analysis of p-STAT3+ osteocyte, RANKL expression, and bone formation/resorption markers.
Results : In vitro, IL-6 dose-dependently elevated RANKL and p-STAT3 levels in osteocyte and promoted osteoclast activity in co-culture. These effects were suppressed by Stattic and replicated by STAT3 knockdown. In contrast, co-culture of osteocyte with osteoblast exhibited no significant alterations in osteogenic marker expression upon IL-6 exposure, suggesting negligible effects on osteoblast activity. In vivo, MR16-1 reduced trabecular bone loss in the vertebrae and femur after fracture. It also diminished p-STAT3+ osteocyte, reduced RANKL expression, and suppressed osteoclast activity without impairing osteoblastogenesis. And Stattic produced a comparable reduction in systemic bone loss and osteoclast overactivation.
Conclusion: This study demonstrates that IL-6 drives osteoclast-mediated bone resorption via STAT3-dependent RANKL induction in osteocyte, thereby aggravating post-fracture systemic bone loss. And the findings highlight that modulating the IL-6/STAT3/RANKL axis and targeting osteocyte function may offer a promising therapeutic approach for preventing bone loss and minimizing the risk of fracture recurrence.
Keywords: RANKL; fracture healing; fracture risk; inflammation; osteocyte; osteoporosis.
© 2025 Sun et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures







Similar articles
-
An herbal formula inhibits STAT3 signaling and attenuates bone erosion in collagen-induced arthritis rats.Phytomedicine. 2020 Sep;76:153254. doi: 10.1016/j.phymed.2020.153254. Epub 2020 May 30. Phytomedicine. 2020. PMID: 32531698
-
Chk2 deletion rescues bone loss and cellular senescence induced by Bmi1 deficiency via regulation of Cyp1a1.J Orthop Translat. 2025 May 10;52:360-375. doi: 10.1016/j.jot.2025.04.014. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698069 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Treatment for osteoporosis in people with beta-thalassaemia.Cochrane Database Syst Rev. 2023 May 9;5(5):CD010429. doi: 10.1002/14651858.CD010429.pub3. Cochrane Database Syst Rev. 2023. PMID: 37159055 Free PMC article.
-
Rehabilitation for ankle fractures in adults.Cochrane Database Syst Rev. 2024 Sep 23;9(9):CD005595. doi: 10.1002/14651858.CD005595.pub4. Cochrane Database Syst Rev. 2024. PMID: 39312389
References
LinkOut - more resources
Full Text Sources
Miscellaneous

