Hydrothermal synthesis of Fe2O3-Ag2O nanocomposites supported on pristine, N/S doped graphene oxide for photocatalytic and biological applications
- PMID: 40860422
- PMCID: PMC12376950
- DOI: 10.1039/d5ra05475c
Hydrothermal synthesis of Fe2O3-Ag2O nanocomposites supported on pristine, N/S doped graphene oxide for photocatalytic and biological applications
Abstract
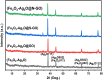
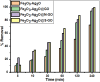
Addressing the dual challenges of industrial contamination in wastewater and antimicrobial resistance is crucial for environmental preservation and global health, aligning with sustainable development goals. This study investigated the biological and photocatalytic potential of pure bimetal oxides, nanocomposites (NCs) and their counterparts incorporating graphene oxide (GO) and nitrogen- and sulfur-doped GO. A set of samples, namely Fe2O3-Ag2O, Fe2O3-Ag2O@GO, Fe2O3-Ag2O@N-GO, and Fe2O3-Ag2O@S-GO NCs, was synthesized using the hydrothermal method. The synthesized NCs underwent comprehensive structural and compositional analyses using XRD, FTIR, EDX, SEM, and UV-visible spectroscopy. Their photocatalytic performance was evaluated for methylene blue (MB) dye degradation under direct sunlight at varying pH levels, dye concentrations, and reaction times. The photocatalytic tests revealed a significant enhancement in methylene blue (MB) degradation efficiency, which generally increased with rising pH, albeit with some deviations. Among all samples, Fe2O3-Ag2O@S-GO NC exhibited the highest photocatalytic activity, achieving maximum degradation at 240 min. The biological properties of NCs were assessed through protein kinase inhibition studies and antimicrobial assays, while hemocompatibility was determined via hemolytic activity. All samples exhibited both bacteriostatic and bactericidal effects, as indicated by the presence of a bald zone alongside a clear zone. The Fe2O3-Ag2O NC demonstrated the most notable antibacterial activity, achieving the MIC of 12.5 against S. aureus. The antibacterial assay further demonstrated enhanced inhibitory effects against both resistant and non-resistant bacterial strains, confirming that iron- and silver-doped composites exhibited superior antibacterial properties. Among all the NCs, Fe2O3-Ag2O@GO NC displayed significant antifungal activity against five different strains, with the highest effectiveness against Aspergillus niger and Mucor, achieving a zone of inhibition of 9 ± 0.01 mm. The hemolytic assay indicated that all NCs exhibited hemolysis levels below 1%, confirming their safety for biological applications. This study highlighted the potential of these NCs for enhancing the photocatalytic degradation of MB dye in water while also demonstrating their biological significance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Ce-doped nano-Fe-TiO2 composites for photocatalytic degradation of naphthol blue black in aqueous medium.RSC Adv. 2025 Jul 17;15(31):25322-25336. doi: 10.1039/d5ra02583d. eCollection 2025 Jul 15. RSC Adv. 2025. PMID: 40677944 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
One-step co-precipitation synthesis, characterization, and enhanced photocatalytic performance of CaO/TiO2-supported γ-Al2O3 nanocomposites (NCs) in wastewater treatment.RSC Adv. 2025 Jul 15;15(30):24851-24861. doi: 10.1039/d5ra03493k. eCollection 2025 Jul 10. RSC Adv. 2025. PMID: 40666418 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Smith R. Y. Kar A. Subramanian V. Ind. Eng. Chem. Res. 2019;48:10268–10276. doi: 10.1021/ie801851p. - DOI
-
- Farhan Hanafi M. Sapawe N. Mater. Today: Proc. 2020;31:A141–A150.
-
- Zhang G. Zhang X. Meng Y. Pan G. Ni Z. Xia S. Chem. Eng. J. 2019:123684.
-
- Lin C. Liu H. Guo M. Zhao Y. Su X. Zhang P. Zhang Y. Colloids Surf., A. 2022;646:128962. doi: 10.1016/j.colsurfa.2022.128962. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

